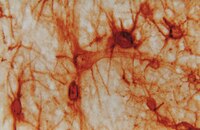
A Rac/Cdc42 exchange factor complex promotes formation of lateral filopodia and blood vessel lumen morphogenesis.
Abraham, S; Scarcia, M; Bagshaw, RD; McMahon, K; Grant, G; Harvey, T; Yeo, M; Esteves, FO; Thygesen, HH; Jones, PF; Speirs, V; Hanby, AM; Selby, PJ; Lorger, M; Dear, TN; Pawson, T; Marshall, CJ; Mavria, G
Nature communications
6
7286
2015
Show Abstract
During angiogenesis, Rho-GTPases influence endothelial cell migration and cell-cell adhesion; however it is not known whether they control formation of vessel lumens, which are essential for blood flow. Here, using an organotypic system that recapitulates distinct stages of VEGF-dependent angiogenesis, we show that lumen formation requires early cytoskeletal remodelling and lateral cell-cell contacts, mediated through the RAC1 guanine nucleotide exchange factor (GEF) DOCK4 (dedicator of cytokinesis 4). DOCK4 signalling is necessary for lateral filopodial protrusions and tubule remodelling prior to lumen formation, whereas proximal, tip filopodia persist in the absence of DOCK4. VEGF-dependent Rac activation via DOCK4 is necessary for CDC42 activation to signal filopodia formation and depends on the activation of RHOG through the RHOG GEF, SGEF. VEGF promotes interaction of DOCK4 with the CDC42 GEF DOCK9. These studies identify a novel Rho-family GTPase activation cascade for the formation of endothelial cell filopodial protrusions necessary for tubule remodelling, thereby influencing subsequent stages of lumen morphogenesis. | | | 26129894
 |
3-D Imaging Reveals Participation of Donor Islet Schwann Cells and Pericytes in Islet Transplantation and Graft Neurovascular Regeneration.
Juang, JH; Kuo, CH; Peng, SJ; Tang, SC
EBioMedicine
2
109-19
2015
Show Abstract
The primary cells that participate in islet transplantation are the endocrine cells. However, in the islet microenvironment, the endocrine cells are closely associated with the neurovascular tissues consisting of the Schwann cells and pericytes, which form sheaths/barriers at the islet exterior and interior borders. The two cell types have shown their plasticity in islet injury, but their roles in transplantation remain unclear. In this research, we applied 3-dimensional neurovascular histology with cell tracing to reveal the participation of Schwann cells and pericytes in mouse islet transplantation. Longitudinal studies of the grafts under the kidney capsule identify that the donor Schwann cells and pericytes re-associate with the engrafted islets at the peri-graft and perivascular domains, respectively, indicating their adaptability in transplantation. Based on the morphological proximity and cellular reactivity, we propose that the new islet microenvironment should include the peri-graft Schwann cell sheath and perivascular pericytes as an integral part of the new tissue. | | | 26137552
 |
Traumatic brain injury results in rapid pericyte loss followed by reactive pericytosis in the cerebral cortex.
Zehendner, CM; Sebastiani, A; Hugonnet, A; Bischoff, F; Luhmann, HJ; Thal, SC
Scientific reports
5
13497
2015
Show Abstract
Accumulating evidence suggests a pivotal role of PDGFRß positive cells, a specific marker for central nervous system (CNS) pericytes, in tissue scarring. Identification of cells that contribute to tissue reorganization in the CNS upon injury is a crucial step to develop novel treatment strategies in regenerative medicine. It has been shown that pericytes contribute to scar formation in the spinal cord. It is further known that ischemia initially triggers pericyte loss in vivo, whilst brain trauma is capable of inducing pericyte detachment from cerebral vessels. These data point towards a significant role of pericytes in CNS injury. The temporal and spatial dynamics of PDGFRß cells and their responses in traumatic brain injury are poorly understood. Here we show that PDGFRß positive cells initially decline in the acute phase following experimental traumatic brain injury. However, PDGFRß positive cells increase significantly in the trauma zone days after brain injury. Using various pericyte markers we identify these cells to be pericytes that are demarcated by reactive gliosis. Our data indicate that brain trauma causes a biphasic response of pericytes in the early phase of brain trauma that may be of relevance for the understanding of pathological cellular responses in traumatic brain injury. | | | 26333872
 |
Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors.
Gautier, HO; Evans, KA; Volbracht, K; James, R; Sitnikov, S; Lundgaard, I; James, F; Lao-Peregrin, C; Reynolds, R; Franklin, RJ; Káradóttir, RT
Nature communications
6
8518
2015
Show Abstract
Myelin regeneration can occur spontaneously in demyelinating diseases such as multiple sclerosis (MS). However, the underlying mechanisms and causes of its frequent failure remain incompletely understood. Here we show, using an in-vivo remyelination model, that demyelinated axons are electrically active and generate de novo synapses with recruited oligodendrocyte progenitor cells (OPCs), which, early after lesion induction, sense neuronal activity by expressing AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid)/kainate receptors. Blocking neuronal activity, axonal vesicular release or AMPA receptors in demyelinated lesions results in reduced remyelination. In the absence of neuronal activity there is a ∼6-fold increase in OPC number within the lesions and a reduced proportion of differentiated oligodendrocytes. These findings reveal that neuronal activity and release of glutamate instruct OPCs to differentiate into new myelinating oligodendrocytes that recover lost function. Co-localization of OPCs with the presynaptic protein VGluT2 in MS lesions implies that this mechanism may provide novel targets to therapeutically enhance remyelination. | | | 26439639
 |
Interneurons and oligodendrocyte progenitors form a structured synaptic network in the developing neocortex.
Orduz, D; Maldonado, PP; Balia, M; Vélez-Fort, M; de Sars, V; Yanagawa, Y; Emiliani, V; Angulo, MC
eLife
4
2015
Show Abstract
NG2 cells, oligodendrocyte progenitors, receive a major synaptic input from interneurons in the developing neocortex. It is presumed that these precursors integrate cortical networks where they act as sensors of neuronal activity. We show that NG2 cells of the developing somatosensory cortex form a transient and structured synaptic network with interneurons that follows its own rules of connectivity. Fast-spiking interneurons, highly connected to NG2 cells, target proximal subcellular domains containing GABAA receptors with γ2 subunits. Conversely, non-fast-spiking interneurons, poorly connected with these progenitors, target distal sites lacking this subunit. In the network, interneuron-NG2 cell connectivity maps exhibit a local spatial arrangement reflecting innervation only by the nearest interneurons. This microcircuit architecture shows a connectivity peak at PN10, coinciding with a switch to massive oligodendrocyte differentiation. Hence, GABAergic innervation of NG2 cells is temporally and spatially regulated from the subcellular to the network level in coordination with the onset of oligodendrogenesis. | | | 25902404
 |
Cytogenesis in the adult monkey motor cortex: perivascular NG2 cells are the major adult born cell type.
Stanton, GB; Kohler, SJ; Boklweski, J; Cameron, JL; Greenough, WT
The Journal of comparative neurology
523
849-68
2015
Show Abstract
We used confocal microscopy and immunohistochemistry (IHC) to look for new cells in the motor cortex of adult macaque monkeys that might form the cellular bases of improved brain function from exercise. Twenty-four female Macaca fascicularis monkeys divided into groups by age (10-12 years, 15-17 years), postexercise survival periods, and controls, received 10 weekly injections of the thymidine analog, bromodeoxyuridine (BrdU) to mark new cells. Sixteen monkeys survived 15 weeks (5 weeks postexercise) and 8 monkeys survived 27 weeks (12 weeks postexercise) after initial BrdU injections. Additionally, five Macaca mulatta female monkeys (∼5.5-7 years) received single injections of BrdU and survived 2 days, 2 weeks, and 6 weeks after BrdU injections. Neural and glial antibodies were used to identify new cell phenotypes and to look for changes in proportions of these cells with respect to time and experimental conditions. No BrdU(+) /DCx(+) cells were found but about 7.5% of new cells were calretinin-positive (Cr(+) ). BrdU(+) /GABA(+) (gamma-aminobutyric acid) cells were also found but no new Cr(+) or GABA(+) cells colabeled with a mature neuron marker, NeuN or chondroitin sulfate antibody, NG2. The proportion of new cells that were NG2(+) was about 85% for short and long survival monkeys of which two, newly described perivascular phenotypes (Pldv and Elu) and a small percentage of pericytes (2.5%) comprised 44% and 51% of the new NG2(+) cells, respectively. Proportions of NG2(+) phenotypes were affected by post-BrdU survival periods, monkey age, and possibly a postexercise sedentary period but no direct effect of exercise was found. | | | 25308320
 |
Systemic inflammation in early neonatal mice induces transient and lasting neurodegenerative effects.
Cardoso, FL; Herz, J; Fernandes, A; Rocha, J; Sepodes, B; Brito, MA; McGavern, DB; Brites, D
Journal of neuroinflammation
12
82
2015
Show Abstract
The inflammatory mediator lipopolysaccharide (LPS) has been shown to induce acute gliosis in neonatal mice. However, the progressive effects on the murine neurodevelopmental program over the week that follows systemic inflammation are not known. Thus, we investigated the effects of repeated LPS administration in the first postnatal week in mice, a condition mimicking sepsis in late preterm infants, on the developing central nervous system (CNS).Systemic inflammation was induced by daily intraperitoneal administration (i.p.) of LPS (6 mg/kg) in newborn mice from postnatal day (PND) 4 to PND6. The effects on neurodevelopment were examined by staining the white matter and neurons with Luxol Fast Blue and Cresyl Violet, respectively. The inflammatory response was assessed by quantifying the expression/activity of matrix metalloproteinases (MMP), toll-like receptor (TLR)-4, high mobility group box (HMGB)-1, and autotaxin (ATX). In addition, B6 CX3CR1(gfp/+) mice combined with cryo-immunofluorescence were used to determine the acute, delayed, and lasting effects on myelination, microglia, and astrocytes.LPS administration led to acute body and brain weight loss as well as overt structural changes in the brain such as cerebellar hypoplasia, neuronal loss/shrinkage, and delayed myelination. The impaired myelination was associated with alterations in the proliferation and differentiation of NG2 progenitor cells early after LPS administration, rather than with excessive phagocytosis by CNS myeloid cells. In addition to disruptions in brain architecture, a robust inflammatory response to LPS was observed. Quantification of inflammatory biomarkers revealed decreased expression of ATX with concurrent increases in HMGB1, TLR-4, and MMP-9 expression levels. Acute astrogliosis (GFAP(+) cells) in the brain parenchyma and at the microvasculature interface together with parenchymal microgliosis (CX3CR1(+) cells) were also observed. These changes preceded the migration/proliferation of CX3CR1(+) cells around the vessels at later time points and the subsequent loss of GFAP(+) astrocytes.Collectively, our study has uncovered a complex innate inflammatory reaction and associated structural changes in the brains of neonatal mice challenged peripherally with LPS. These findings may explain some of the neurobehavioral abnormalities that develop following neonatal sepsis. | | | 25924675
 |
The adhesion G protein-coupled receptor GPR56 is a cell-autonomous regulator of oligodendrocyte development.
Giera, S; Deng, Y; Luo, R; Ackerman, SD; Mogha, A; Monk, KR; Ying, Y; Jeong, SJ; Makinodan, M; Bialas, AR; Chang, BS; Stevens, B; Corfas, G; Piao, X
Nature communications
6
6121
2015
Show Abstract
Mutations in GPR56, a member of the adhesion G protein-coupled receptor family, cause a human brain malformation called bilateral frontoparietal polymicrogyria (BFPP). Magnetic resonance imaging (MRI) of BFPP brains reveals myelination defects in addition to brain malformation. However, the cellular role of GPR56 in oligodendrocyte development remains unknown. Here, we demonstrate that loss of Gpr56 leads to hypomyelination of the central nervous system in mice. GPR56 levels are abundant throughout early stages of oligodendrocyte development, but are downregulated in myelinating oligodendrocytes. Gpr56-knockout mice manifest with decreased oligodendrocyte precursor cell (OPC) proliferation and diminished levels of active RhoA, leading to fewer mature oligodendrocytes and a reduced number of myelinated axons in the corpus callosum and optic nerves. Conditional ablation of Gpr56 in OPCs leads to a reduced number of mature oligodendrocytes as seen in constitutive knockout of Gpr56. Together, our data define GPR56 as a cell-autonomous regulator of oligodendrocyte development. | | | 25607655
 |
C/EBPβ expression is an independent predictor of overall survival in breast cancer patients by MHCII/CD4-dependent mechanism of metastasis formation.
Kurzejamska, E; Johansson, J; Jirström, K; Prakash, V; Ananthaseshan, S; Boon, L; Fuxe, J; Religa, P
Oncogenesis
3
e125
2014
Show Abstract
CCAAT-enhancer binding protein β (C/EBPβ) is a transcription factor that has a critical role in mammary gland development and breast cancer progression. Loss of C/EBPβ increases metastatic dissemination of mouse mammary tumor cells. However, the mechanism by which C/EBPβ expression affects metastasis formation remains unknown. This study aims at determining the relationship between C/EBPβ and survival of breast cancer patients, and elucidating C/EBPβ's link with metastasis formation. C/EBPβ expression was evaluated in 137 cases of human breast cancer, and the correlation with overall survival was estimated by Kaplan-Meier analysis. Additionally, the mouse 4T1 tumor model was used for in vivo studies. Decreased C/EBPβ expression was found to be associated with shorter overall survival of breast cancer patients. In the murine 4T1 model, loss of C/EBPβ affects tumor growth, morphology and promotes metastatic spread to the lungs. Immunohistochemical analyses showed that C/EBPβ inhibition leads to increased major histocompatibility complex II (MHCII) expression, followed by the accumulation of CD45-, CD3- and CD4-positive (CD4+) lymphocytes in the tumors. Inflammation involvement in C/EBPβ-mediated metastasis formation was confirmed by DNA microarray and by experiments on CD4+ cell-deprived nude mice. Additionally, anti-CD3 and anti-CD4 treatments of C/EBPβ-silenced tumor-bearing mice resulted in reverting the C/EBPβ effect on tumor growth and metastasis. Altogether, C/EBPβ is a predictor of overall survival in breast cancer patients, and affects tumor growth, morphology and lung metastasis formation in murine 4T1 model. The mechanism of metastasis formation involves immunologic response depending on C/EBPβ-mediated activation of MHCII and accumulation of CD4+ lymphocytes in the tumor. | | | 25365481
 |
A competitive advantage by neonatally engrafted human glial progenitors yields mice whose brains are chimeric for human glia.
Windrem, MS; Schanz, SJ; Morrow, C; Munir, J; Chandler-Militello, D; Wang, S; Goldman, SA
The Journal of neuroscience : the official journal of the Society for Neuroscience
34
16153-61
2014
Show Abstract
Neonatally transplanted human glial progenitor cells (hGPCs) densely engraft and myelinate the hypomyelinated shiverer mouse. We found that, in hGPC-xenografted mice, the human donor cells continue to expand throughout the forebrain, systematically replacing the host murine glia. The differentiation of the donor cells is influenced by the host environment, such that more donor cells differentiated as oligodendrocytes in the hypomyelinated shiverer brain than in myelin wild-types, in which hGPCs were more likely to remain as progenitors. Yet in each recipient, both the number and relative proportion of mouse GPCs fell as a function of time, concomitant with the mitotic expansion and spread of donor hGPCs. By a year after neonatal xenograft, the forebrain GPC populations of implanted mice were largely, and often entirely, of human origin. Thus, neonatally implanted hGPCs outcompeted and ultimately replaced the host population of mouse GPCs, ultimately generating mice with a humanized glial progenitor population. These human glial chimeric mice should permit us to define the specific contributions of glia to a broad variety of neurological disorders, using human cells in vivo. | | | 25429155
 |