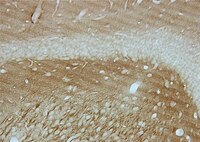
Neuropathologic analysis of Tyr69His TTR variant meningovascular amyloidosis with dementia.
Ziskin, JL; Greicius, MD; Zhu, W; Okumu, AN; Adams, CM; Plowey, ED
Acta neuropathologica communications
3
43
2015
Show Abstract
Transthyretin/TTR gene mutations usually cause systemic amyloidotic diseases. Few TTR variants preferentially affect the central nervous system, manifesting as oculoleptomeningeal amyloidosis. Patients with TTR meningovascular amyloidosis often show dementia, however the neuropathologic features of dementia in these cases have not been elucidated. We report the neuropathologic findings from a brain autopsy of a 72-year-old man with the rare Tyr69His (Y69H) TTR gene variant, dementia and ataxia. Severe amyloid deposits were observed in the leptomeninges and in a subpial and subependymal distribution. Mass spectrometry analysis demonstrated that the amyloid deposits were comprised of over 80 % of the variant TTR. TTR was undetectable by mass spectrometry in the neocortex subjacent to the subpial amyloid deposits. Subpial TTR amyloid deposits were associated with brisk superficial reactive gliosis and siderosis in the neocortex and cerebellar cortex. Subependymal TTR amyloid deposits were associated with subjacent myelin pallor in the hippocampal outflow tract structures including the alveus, fimbria and fornix. Phospho-tau immunostains demonstrated transentorhinal-stage neurofibrillary degeneration (Braak stage II) which, in the absence of neocortical amyloid-beta and neuritic plaques, was indicative of primary age-related tauopathy (PART). However, distinctive phospho-tau aggregates were observed subjacent to the subpial TTR amyloid deposits in all regions of the neocortex, including the primary motor and striate cortices, suggesting a potential link between TTR amyloid and neocortical tauopathy. Our report reveals novel insights into the potential neuropathologic substrates of dementia in variant TTR amyloidosis that need to be investigated in larger autopsy series. | 26156087
 |
Nuclear export inhibitors avert progression in preclinical models of inflammatory demyelination.
Haines, JD; Herbin, O; de la Hera, B; Vidaurre, OG; Moy, GA; Sun, Q; Fung, HY; Albrecht, S; Alexandropoulos, K; McCauley, D; Chook, YM; Kuhlmann, T; Kidd, GJ; Shacham, S; Casaccia, P
Nature neuroscience
18
511-20
2015
Show Abstract
Axonal damage has been associated with aberrant protein trafficking. We examined a newly characterized class of compounds that target nucleo-cytoplasmic shuttling by binding to the catalytic groove of the nuclear export protein XPO1 (also known as CRM1, chromosome region maintenance protein 1). Oral administration of reversible CRM1 inhibitors in preclinical murine models of demyelination significantly attenuated disease progression, even when started after the onset of paralysis. Clinical efficacy was associated with decreased proliferation of immune cells, characterized by nuclear accumulation of cell cycle inhibitors, and preservation of cytoskeletal integrity even in demyelinated axons. Neuroprotection was not limited to models of demyelination, but was also observed in another mouse model of axonal damage (that is, kainic acid injection) and detected in cultured neurons after knockdown of Xpo1, the gene encoding CRM1. A proteomic screen for target molecules revealed that CRM1 inhibitors in neurons prevented nuclear export of molecules associated with axonal damage while retaining transcription factors modulating neuroprotection. | 25706475
 |
Profiling murine tau with 0N, 1N and 2N isoform-specific antibodies in brain and peripheral organs reveals distinct subcellular localization, with the 1N isoform being enriched in the nucleus.
Liu, C; Götz, J
PloS one
8
e84849
2013
Show Abstract
In the adult murine brain, the microtubule-associated protein tau exists as three major isoforms, which have four microtubule-binding repeats (4R), with either no (0N), one (1N) or two (2N) amino-terminal inserts. The human brain expresses three additional isoforms with three microtubule-binding repeats (3R) each. However, little is known about the role of the amino-terminal inserts and how the 0N, 1N and 2N tau species differ. In order to investigate this, we generated a series of isoform-specific antibodies and performed a profiling by Western blotting and immunohistochemical analyses using wild-type mice in three age groups: two months, two weeks and postnatal day 0 (P0). This revealed that the brain is the only organ to express tau at significant levels, with 0N4R being the predominant isoform in the two month-old adult. Subcellular fractionation of the brain showed that the 1N isoform is over-represented in the soluble nuclear fraction. This is in agreement with the immunohistochemical analysis as the 1N isoform strongly localizes to the neuronal nucleus, although it is also found in cell bodies and dendrites, but not axons. The 0N isoform is mainly found in cell bodies and axons, whereas nuclei and dendrites are only slightly stained with the 0N antibody. The 2N isoform is highly expressed in axons and in cell bodies, with a detectable expression in dendrites and a very slight expression in nuclei. The 2N isoform that was undetectable at P0, in adult brain was mainly found localized to cell bodies and dendrites. Together these findings reveal significant differences between the three murine tau isoforms that are likely to reflect different neuronal functions. | 24386422
 |
Expression of the embryonal isoform (0N/3R) of the microtubule-associated protein tau in the adult rat central nervous system.
Torsten Bullmann,Wolfgang Härtig,Max Holzer,Thomas Arendt
The Journal of comparative neurology
518
2010
Show Abstract
Tau is a microtubule-associated protein expressed predominantly in neurons. The transcript of the tau gene is alternatively spliced. Resulting isoforms contain three or four microtubule-binding repeats. The shortest tau isoform contains only three repeats (3R) and is expressed at birth. Previous data on rodents suggested that this isoform is no longer expressed during adulthood. It is replaced by tau isoforms containing four repeats (4R). The adult 4R tau isoforms bind to microtubules with higher affinity than 3R tau isoforms. Therefore, this isoform switch may reflect a need for more dynamic microtubules during development. Recently, we observed in rats that the 3R tau isoform is transiently expressed in adult neurogenesis. Subsequently, we performed an immunohistochemical labeling of the 3R tau isoform on serial sections of the adult rat brain. Interestingly, the 3R tau isoform is not only expressed in neuronal precursor cells. It is also present in mature neurons of the olfactory bulb, magnocellular neurosecretory system, posterolateral hypothalamus, locus coeruleus, raphe nucleus, solitary nucleus, medial septum and diagonal band, olfactory tuberculus, and piriform/olfactory cortex. This expression pattern is similar to that observed for the polysialylated form of the neuronal cell adhesion molecule (PSA-NCAM) and the microtubule-associated proteins doublecortin and collapsin response mediating protein (CRMP-4/TUC-4/Ulip-1), which are also highly expressed during early development. The retention of a juvenile phenotype in some neurons might be associated with a functionally significant neuronal plasticity. | 20503426
 |
Differential involvement and heterogeneous phosphorylation of tau isoforms in progressive supranuclear palsy.
Gibb, G M, et al.
Brain Res. Mol. Brain Res., 121: 95-101 (2004)
2004
Show Abstract
We found previously that aggregated insoluble tau protein in progressive supranuclear palsy (PSP) brains exhibits a heterogeneous pattern that is not segregated by the type of clinical presentation. Here we have investigated tau isoform composition from 20 PSP cases and found marked variation between different brains. Cases were classified into three groups, each comprising essentially of (1) 1N4R; (2) 1N4R and 1N3R; or (3) 1N4R, 1N3R and 0N4R tau isoforms. There was also an absence of a simple relationship between isoform composition and the pattern of insoluble tau before dephosphorylation. We conclude that there is distinct molecular heterogeneity in the involvement of tau isoforms in the tau pathology in PSP. | 14969740
 |
Dementia with Lewy bodies from the perspective of tauopathy.
Iseki, Eizo, et al.
Acta Neuropathol., 105: 265-70 (2003)
2003
Show Abstract
We immunohistochemically investigated the prevalence and pattern of phosphorylated tau accumulation in neurons and glia in 46 cases of dementia with Lewy bodies (DLB). Tau-positive neurons composed of neurofibrillary tangles (NFT) and pretangle neurons were found in the hippocampal area in all 46 cases, although the ratio of pretangle neurons in tau-positive neurons was higher in the cases showing low NFT stages. Tau-positive astrocytes were found in the periventricular area in 18 of 46 cases, and partly represented argyrophilic thorn-shaped astrocytes. In contrast, tau-positive oligodendroglia were found in the subcortical white matter in 9 of 46 cases, and represented argyrophilic coiled bodies. Tau-positive argyrophilic grains were found in the hippocampal area in the same cases as those with coiled bodies. The 9 cases with tau-positive coiled bodies and grains were included in the 18 cases with tau-positive astrocytes, and showed larger proportions in the low NFT stages than the 46 cases with tau-positive neurons. Tau-positive neurons were positive both to anti-three-repeat (3R) and -4R tau-specific antibodies, while tau-positive astrocytes, coiled bodies and grains were predominantly positive to anti-4R tau-specific antibody. These tau-positive structures were negative to anti-alpha-synuclein antibody. These findings suggest that the tau accumulation in DLB represents both tau-positive neurons with all six tau isoforms and tau-positive astrocytes, coiled bodies and grains with the 4R tau isoform, and that the different cytoskeletal abnormalities form a link between some neurodegenerative dementing disorders including DLB. | 12557014
 |
The L266V tau mutation is associated with frontotemporal dementia and Pick-like 3R and 4R tauopathy.
Hogg, Marion, et al.
Acta Neuropathol., 106: 323-36 (2003)
2003
Show Abstract
We report a case of rapidly progressive frontotemporal dementia presenting at age 33 years. At autopsy there was severe atrophy of the frontal and temporal lobes. Tau-positive Pick bodies, which ultrastructurally were composed of straight filaments, were present, accompanied by severe neuronal loss and gliosis. RD3, a tau antibody specific for the three-repeat (3R) isoforms, labeled the Pick bodies. ET3, a four-repeat (4R) isoform-specific tau antibody, did not label Pick bodies, but highlighted rare astrocytes, and threads in white matter bundles in the corpus striatum. Analysis of the tau gene revealed an L266V mutation in exon 9. Analysis of brain tissue from this case revealed elevated levels of exon 10+ tau RNA and soluble 4R tau. However, both 3R and 4R isoforms were present in sarkosyl-insoluble tau fractions with a predominance of the shortest 3R isoform. The L266V mutation is associated with decreased rate and extent of tau-induced microtubule assembly, and a 3R isoform-specific increase in tau self assembly as measured by an in vitro assay. Combined, these data indicate that L266V is a pathogenic tau mutation that is associated with Pick-like pathology. In addition, the results of the RD3 and ET3 immunostains clearly explain for the first time the presence of both 3R and 4R tau isoforms in preparations of insoluble tau from some Pick's disease cases. | 12883828
 |
Argyrophilic grain disease is a sporadic 4-repeat tauopathy
Togo, Takashi, et al
J Neuropathol Exp Neurol, 61:547-56 (2002)
2002
| 12071638
 |
The slow axonal transport of the microtubule-associated protein tau and the transport rates of different isoforms and mutants in cultured neurons.
Utton, Michelle A, et al.
J. Neurosci., 22: 6394-400 (2002)
2002
Show Abstract
We demonstrate that the microtubule-associated protein tau, in the form of enhanced green fluorescent protein (EGFP) tau, is transported along axons of neurons in culture in the slow component of axonal transport with a speed comparable with that previously measured in vivo. It was demonstrated that the EGFP tag has no effect on transport characteristics, and the methodology enables slow transport rates of individual tau isoforms and tau mutants to be measured. We also expressed EGFP-tagged tau isoforms containing either three or four C-terminal repeats and zero or two N-terminal inserts in cultured neurons. No significant differences were found in the average rate of slow transport of the wild-type tau isoforms, suggesting that the exon 10 C-terminal repeat or the N-terminal inserts do not contain regions that play a significant regulatory role in axonal transport. Similarly, we found that missense mutations in tau have no noticeable effect on the rate of transport; hence their ability to cause neurodegeneration is by another mechanism other than that affecting the overall slow axonal transport of tau. | 12151518
 |