RE-IIBP Methylates H3K79 and Induces MEIS1-mediated Apoptosis via H2BK120 Ubiquitination by RNF20.
Woo Park, J; Kim, KB; Kim, JY; Chae, YC; Jeong, OS; Seo, SB
Scientific reports
5
12485
2015
Show Abstract
Histone lysine methylation contributes to transcriptional regulation by serving as a platform for the recruitment of various cofactors. Intense studies have been conducted for elucidating the functional meaning of H3K79 methylation, and to date, the only known HMTase responsible for the modification was DOT1L. In this study, we report that the MMSET isoform RE-IIBP has HMTase activity for H3K79. It was uncovered that RE-IIBP up-regulates MEIS1 transcription through H3K79 methylation via recruitment to the MEIS1 promoter. By means of proteomic and biochemical analysis, association of RE-IIBP with the E3 ubiquitin ligase RNF20 was demonstrated for synergistic activation of MEIS1 transcription via H3K79 HMTase activity. Furthermore, It was observed that RE-IIBP induces MEIS1-mediated apoptosis, which was dependent on H2BK120 ubiquitination by RNF20. These findings suggest RE-IIBP as another candidate for further studies to elucidate the mechanism of H3K79 methylation and its biological functions. | | | 26206755
 |
Cytomixis doesn't induce obvious changes in chromatin modifications and programmed cell death in tobacco male meiocytes.
Mursalimov, S; Permyakova, N; Deineko, E; Houben, A; Demidov, D
Frontiers in plant science
6
846
2015
Show Abstract
Cytomixis is a poorly studied process of nuclear migration between plant cells. It is so far unknown what drives cytomixis and what is the functional state of the chromatin migrating between cells. Using immunostaining, we have analyzed the distribution of posttranslational histone modifications (methylation, acetylation, and phosphorylation) that reflect the functional state of chromatin in the tobacco microsporocytes involved in cytomixis. We demonstrate that the chromatin in the cytomictic cells does not differ from the chromatin in intact microsporocytes according to all 14 analyzed histone modification types. We have also for the first time demonstrated that the migrating chromatin contains normal structures of the synaptonemal complex (SC) and lacks any signs of apoptosis. As has been shown, the chromatin migrating between cells in cytomixis is neither selectively heterochromatized nor degraded both before its migration to another cell and after it enters a recipient cell as micronuclei. We also showed that cytomictic chromatin contains marks typical for transcriptionally active chromatin as well as heterochromatin. Moreover, marks typical for chromosome condensation, SC formation and key proteins required for the formation of bivalents were also detected at migrated chromatin. | | | 26528310
 |
Epigenetic regulation of traf2- and Nck-interacting kinase (TNIK) in polycystic ovary syndrome.
Li, D; Jiao, J; Zhou, YM; Wang, XX
American journal of translational research
7
1152-60
2015
Show Abstract
Emerging evidence has led to considerable interest in the role of Traf2- and Nck-interacting kinase (TNIK) in polycystic ovary syndrome (PCOS) development. However, the epigenetic mechanism regulating TNIK transcription remains largely unknown. Here, we show that (i) TNIK mRNA expression is significantly increased in PCOS ovarian tissues, compared to normal ovarian tissues; (ii) PCOS ovarian tissues exhibit a hypermethylation pattern at the cg10180092 site, (iii) and cg10180092 is the critical site for the transcriptional regulation of TNIK. Mechanistically, hypermethylated cg10180092 site-mediated loss of holocarboxylase synthetase (HLCS)-related H3K9me enrichment activated TNIK transcription in PCOS ovarian tissues. Notably, a substantial body of evidence indicates that DNA hypermethylation is an alternative mechanism for gene inactivation, and a new role for DNA hypermethylationmediated TNIK activating was observed in this study. This may improve our understanding of divergent transcriptional regulation in the initiation and progression of TNIK-related PCOS. | | | 26279758
 |
EZH2 modulates angiogenesis in vitro and in a mouse model of limb ischemia.
Mitić, T; Caporali, A; Floris, I; Meloni, M; Marchetti, M; Urrutia, R; Angelini, GD; Emanueli, C
Molecular therapy : the journal of the American Society of Gene Therapy
23
32-42
2015
Show Abstract
Epigenetic mechanisms may regulate the expression of pro-angiogenic genes, thus affecting reparative angiogenesis in ischemic limbs. The enhancer of zest homolog-2 (EZH2) induces thtrimethylation of lysine 27 on histone H3 (H3K27me3), which represses gene transcription. We explored (i) if EZH2 expression is regulated by hypoxia and ischemia; (ii) the impact of EZH2 on the expression of two pro-angiogenic genes: eNOS and BDNF; (iii) the functional effect of EZH2 inhibition on cultured endothelial cells (ECs); (iv) the therapeutic potential of EZH2 inhibition in a mouse model of limb ischemia (LI). EZH2 expression was increased in cultured ECs exposed to hypoxia (control: normoxia) and in ECs extracted from mouse ischemic limb muscles (control: absence of ischemia). EZH2 increased the H3K27me3 abundance onto regulatory regions of eNOS and BDNF promoters. In vitro RNA silencing or pharmacological inhibition by 3-deazaneplanocin (DZNep) of EZH2 increased eNOS and BDNF mRNA and protein levels and enhanced functional capacities (migration, angiogenesis) of ECs under either normoxia or hypoxia. In mice with experimentally induced LI, DZNep increased angiogenesis in ischaemic muscles, the circulating levels of pro-angiogenic hematopoietic cells and blood flow recovery. Targeting EZH2 for inhibition may open new therapeutic avenues for patients with limb ischemia. | | | 25189741
 |
Stage-dependent and locus-specific role of histone demethylase Jumonji D3 (JMJD3) in the embryonic stages of lung development.
Li, Q; Wang, HY; Chepelev, I; Zhu, Q; Wei, G; Zhao, K; Wang, RF
PLoS genetics
10
e1004524
2014
Show Abstract
Histone demethylases have emerged as important players in developmental processes. Jumonji domain containing-3 (Jmjd3) has been identified as a key histone demethylase that plays a critical role in the regulation of gene expression; however, the in vivo function of Jmjd3 in embryonic development remains largely unknown. To this end, we generated Jmjd3 global and conditional knockout mice. Global deletion of Jmjd3 induces perinatal lethality associated with defective lung development. Tissue and stage-specific deletion revealed that Jmjd3 is dispensable in the later stage of embryonic lung development. Jmjd3 ablation downregulates the expression of genes critical for lung development and function, including AQP-5 and SP-B. Jmjd3-mediated alterations in gene expression are associated with locus-specific changes in the methylation status of H3K27 and H3K4. Furthermore, Jmjd3 is recruited to the SP-B promoter through interactions with the transcription factor Nkx2.1 and the epigenetic protein Brg1. Taken together, these findings demonstrate that Jmjd3 plays a stage-dependent and locus-specific role in the mouse lung development. Our study provides molecular insights into the mechanisms by which Jmjd3 regulates target gene expression in the embryonic stages of lung development. | | | 25079229
 |
The hematopoietic regulator TAL1 is required for chromatin looping between the β-globin LCR and human γ-globin genes to activate transcription.
Yun, WJ; Kim, YW; Kang, Y; Lee, J; Dean, A; Kim, A
Nucleic acids research
42
4283-93
2014
Show Abstract
TAL1 is a key hematopoietic transcription factor that binds to regulatory regions of a large cohort of erythroid genes as part of a complex with GATA-1, LMO2 and Ldb1. The complex mediates long-range interaction between the β-globin locus control region (LCR) and active globin genes, and although TAL1 is one of the two DNA-binding complex members, its role is unclear. To explore the role of TAL1 in transcription activation of the human γ-globin genes, we reduced the expression of TAL1 in erythroid K562 cells using lentiviral short hairpin RNA, compromising its association in the β-globin locus. In the TAL1 knockdown cells, the γ-globin transcription was reduced to 35% and chromatin looping of the (G)γ-globin gene with the LCR was disrupted with decreased occupancy of the complex member Ldb1 and LMO2 in the locus. However, GATA-1 binding, DNase I hypersensitive site formation and several histone modifications were largely maintained across the β-globin locus. In addition, overexpression of TAL1 increased the γ-globin transcription and increased interaction frequency between the (G)γ-globin gene and LCR. These results indicate that TAL1 plays a critical role in chromatin loop formation between the γ-globin genes and LCR, which is a critical step for the transcription of the γ-globin genes. | | | 24470145
 |
Critical role of histone demethylase Jmjd3 in the regulation of CD4+ T-cell differentiation.
Li, Q; Zou, J; Wang, M; Ding, X; Chepelev, I; Zhou, X; Zhao, W; Wei, G; Cui, J; Zhao, K; Wang, HY; Wang, RF
Nature communications
5
5780
2014
Show Abstract
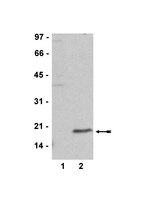
Epigenetic factors have been implicated in the regulation of CD4(+) T-cell differentiation. Jmjd3 plays a role in many biological processes, but its in vivo function in T-cell differentiation remains unknown. Here we report that Jmjd3 ablation promotes CD4(+) T-cell differentiation into Th2 and Th17 cells in the small intestine and colon, and inhibits T-cell differentiation into Th1 cells under different cytokine-polarizing conditions and in a Th1-dependent colitis model. Jmjd3 deficiency also restrains the plasticity of the conversion of Th2, Th17 or Treg cells to Th1 cells. The skewing of T-cell differentiation is concomitant with changes in the expression of key transcription factors and cytokines. H3K27me3 and H3K4me3 levels in Jmjd3-deficient cells are correlated with altered gene expression through interactions with specific transcription factors. Our results identify Jmjd3 as an epigenetic factor in T-cell differentiation via changes in histone methylation and target gene expression. | Western Blotting | | 25531312
 |
Quantitative proteomics reveals that the specific methyltransferases Txr1p and Ezl2p differentially affect the mono-, di- and trimethylation states of histone H3 lysine 27 (H3K27).
Zhang, C; Molascon, AJ; Gao, S; Liu, Y; Andrews, PC
Molecular & cellular proteomics : MCP
12
1678-88
2013
Show Abstract
Nuclear DNA in eukaryotic cells is assembled into the hierarchical chromatin structure via a process that is dynamically affected by the combinatorial set of post-translational modifications (PTMs) of histones in a dynamic manner responsive to physiological and environmental changes. The precise quantification of these complex modifications is challenging. Here we present a robust MS-based quantitative proteomics method for studying histone PTMs using (15)N metabolically labeled histones as the internal reference. Using this approach, we identified Tetrahymena trithorax related 1 (Txr1p) as a histone methyltransferase in Tetrahymena thermophila and characterized the relationships of the Txr1p and Ezl2p methyltransferases to histone H3 modification. We identified 32 PTMs in more than 60 tryptic peptides from histone H3 of the ciliate model organism Tetrahymena thermophila, and we quantified them (average coefficient of variation: 13%). We examined perturbations to histone modification patterns in two knockout strains of SET-domain-containing histone methyltransferases (HMT). Knockout of TXR1 led to progressively decreased mono-, di-, and tri-methylation of H3K27 and apparent reduced monomethylation of H3K36 in vivo. In contrast, EZL2 knockout resulted in dramatic reductions in both di- and tri-methylation of H3K27 in vivo, whereas the levels of monomethylation of H3K27 increased significantly. This buildup of monomethyl H3K27 is consistent with its role as a substrate for Ezl2p. These results were validated via immunoblotting using modification site-specific antibodies. Taken together, our studies define Txr1p as an H3K27 monomethylation-specific HMT that facilitates the buildup of H3K27 di- and trimethylation by the canonical H3K27-specific HMT, Ezl2p. Our studies also delineate some of the interdependences between various H3 modifications, as compensatory increases in monomethylation at H3K4, H3K23, and H3K56 were also observed for both TXR1 and ELZ2 mutants. | Western Blotting | | 23150054
 |
A replication-dependent passive mechanism modulates DNA demethylation in mouse primordial germ cells.
Ohno, R; Nakayama, M; Naruse, C; Okashita, N; Takano, O; Tachibana, M; Asano, M; Saitou, M; Seki, Y
Development (Cambridge, England)
140
2892-903
2013
Show Abstract
Germline cells reprogramme extensive epigenetic modifications to ensure the cellular totipotency of subsequent generations and to prevent the accumulation of epimutations. Notably, primordial germ cells (PGCs) erase genome-wide DNA methylation and H3K9 dimethylation marks in a stepwise manner during migration and gonadal periods. In this study, we profiled DNA and histone methylation on transposable elements during PGC development, and examined the role of DNA replication in DNA demethylation in gonadal PGCs. CpGs in short interspersed nuclear elements (SINEs) B1 and B2 were substantially demethylated in migrating PGCs, whereas CpGs in long interspersed nuclear elements (LINEs), such as LINE-1, were resistant to early demethylation. By contrast, CpGs in both LINE-1 and SINEs were rapidly demethylated in gonadal PGCs. Four major modifiers of DNA and histone methylation, Dnmt3a, Dnmt3b, Glp and Uhrf1, were actively repressed at distinct stages of PGC development. DNMT1 was localised at replication foci in nascent PGCs, whereas the efficiency of recruitment of DNMT1 into replication foci was severely impaired in gonadal PGCs. Hairpin bisulphite sequencing analysis showed that strand-specific hemi-methylated CpGs on LINE-1 were predominant in gonadal PGCs. Furthermore, DNA demethylation in SINEs and LINE-1 was impaired in Cbx3-deficient PGCs, indicating abnormalities in G1 to S phase progression. We propose that PGCs employ active and passive mechanisms for efficient and widespread erasure of genomic DNA methylation. | | | 23760957
 |
Epigenetic regulation of autophagy by the methyltransferase G9a.
Artal-Martinez de Narvajas, A; Gomez, TS; Zhang, JS; Mann, AO; Taoda, Y; Gorman, JA; Herreros-Villanueva, M; Gress, TM; Ellenrieder, V; Bujanda, L; Kim, DH; Kozikowski, AP; Koenig, A; Billadeau, DD
Mol Cell Biol
33
3983-93
2013
Show Abstract
Macroautophagy is an evolutionarily conserved cellular process involved in the clearance of proteins and organelles. Although the cytoplasmic machinery that orchestrates autophagy induction during starvation, hypoxia, or receptor stimulation has been widely studied, the key epigenetic events that initiate and maintain the autophagy process remain unknown. Here we show that the methyltransferase G9a coordinates the transcriptional activation of key regulators of autophagosome formation by remodeling the chromatin landscape. Pharmacological inhibition or RNA interference (RNAi)-mediated suppression of G9a induces LC3B expression and lipidation that is dependent on RNA synthesis, protein translation, and the methyltransferase activity of G9a. Under normal conditions, G9a associates with the LC3B, WIPI1, and DOR gene promoters, epigenetically repressing them. However, G9a and G9a-repressive histone marks are removed during starvation and receptor-stimulated activation of naive T cells, two physiological inducers of macroautophagy. Moreover, we show that the c-Jun N-terminal kinase (JNK) pathway is involved in the regulation of autophagy gene expression during naive-T-cell activation. Together, these findings reveal that G9a directly represses genes known to participate in the autophagic process and that inhibition of G9a-mediated epigenetic repression represents an important regulatory mechanism during autophagy. | | | 23918802
 |