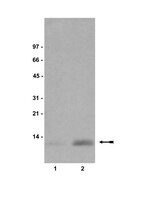
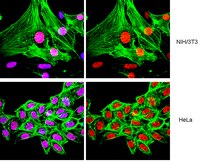
RNF8- and RNF168-dependent degradation of KDM4A/JMJD2A triggers 53BP1 recruitment to DNA damage sites.
Frédérick A Mallette,Francesca Mattiroli,Gaofeng Cui,Leah C Young,Michael J Hendzel,Georges Mer,Titia K Sixma,Stéphane Richard
The EMBO journal
31
2012
Show Abstract
In response to DNA damage, cells initiate complex signalling cascades leading to growth arrest and DNA repair. The recruitment of 53BP1 to damaged sites requires the activation of the ubiquitination cascade controlled by the E3 ubiquitin ligases RNF8 and RNF168, and methylation of histone H4 on lysine 20. However, molecular events that regulate the accessibility of methylated histones, to allow the recruitment of 53BP1 to DNA breaks, are unclear. Here, we show that like 53BP1, the JMJD2A (also known as KDM4A) tandem tudor domain binds dimethylated histone H4K20; however, JMJD2A is degraded by the proteasome following the DNA damage in an RNF8-dependent manner. We demonstrate that JMJD2A is ubiquitinated by RNF8 and RNF168. Moreover, ectopic expression of JMJD2A abrogates 53BP1 recruitment to DNA damage sites, indicating a role in antagonizing 53BP1 for methylated histone marks. The combined knockdown of JMJD2A and JMJD2B significantly rescued the ability of RNF8- and RNF168-deficient cells to form 53BP1 foci. We propose that the RNF8-dependent degradation of JMJD2A regulates DNA repair by controlling the recruitment of 53BP1 at DNA damage sites. | 22373579
 |
PR-Set7 is a nucleosome-specific methyltransferase that modifies lysine 20 of histone H4 and is associated with silent chromatin.
Nishioka, Kenichi, et al.
Mol. Cell, 9: 1201-13 (2002)
2002
Show Abstract
We have purified a human histone H4 lysine 20 methyltransferase and cloned the encoding gene, PR/SET07. A mutation in Drosophila pr-set7 is lethal: second instar larval death coincides with the loss of H4 lysine 20 methylation, indicating a fundamental role for PR-Set7 in development. Transcriptionally competent regions lack H4 lysine 20 methylation, but the modification coincided with condensed chromosomal regions on polytene chromosomes, including chromocenter and euchromatic arms. The Drosophila male X chromosome, which is hyperacetylated at H4 lysine 16, has significantly decreased levels of lysine 20 methylation compared to that of females. In vitro, methylation of lysine 20 and acetylation of lysine 16 on the H4 tail are competitive. Taken together, these results support the hypothesis that methylation of H4 lysine 20 maintains silent chromatin, in part, by precluding neighboring acetylation on the H4 tail. | 12086618
 |