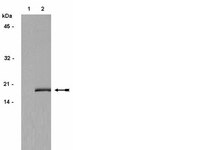
Cell cycle heterogeneity directs the timing of neural stem cell activation from quiescence.
Otsuki L, Brand AH
Science, 360(6384):99-102
2018
Show Abstract
Quiescent stem cells in adult tissues can be activated for homeostasis or repair. Neural stem cells (NSCs) in Drosophila are reactivated from quiescence in response to nutrition by the insulin signaling pathway. It is widely accepted that quiescent stem cells are arrested in G0 In this study, however, we demonstrate that quiescent NSCs (qNSCs) are arrested in either G2 or G0 G2-G0 heterogeneity directs NSC behavior: G2 qNSCs reactivate before G0 qNSCs. In addition, we show that the evolutionarily conserved pseudokinase Tribbles (Trbl) induces G2 NSCs to enter quiescence by promoting degradation of Cdc25String and that it subsequently maintains quiescence by inhibiting Akt activation. Insulin signaling overrides repression of Akt and silences trbl transcription, allowing NSCs to exit quiescence. Our results have implications for identifying and manipulating quiescent stem cells for regenerative purposes. | | | 29622651
 |
A sensitised RNAi screen reveals a ch-TOG genetic interaction network required for spindle assembly.
Barr, AR; Bakal, C
Scientific reports
5
10564
2015
Show Abstract
How multiple spindle assembly pathways are integrated to drive bipolar spindle assembly is poorly understood. We performed an image-based double RNAi screen to identify genes encoding Microtubule-Associated Proteins (MAPs) that interact with the highly conserved ch-TOG gene to regulate bipolar spindle assembly in human cells. We identified a ch-TOG centred network of genetic interactions which promotes centrosome-mediated microtubule polymerisation, leading to the incorporation of microtubules polymerised by all pathways into a bipolar structure [corrected]. Our genetic screen also reveals that ch-TOG maintains a dynamic microtubule population, in part, through modulating HSET activity. ch-TOG ensures that spindle assembly is robust to perturbation but sufficiently dynamic such that spindles can explore a diverse shape space in search of structures that can align chromosomes. | | | 26037491
 |
Neural crest-derived SEMA3C activates endothelial NRP1 for cardiac outflow tract septation.
Plein, A; Calmont, A; Fantin, A; Denti, L; Anderson, NA; Scambler, PJ; Ruhrberg, C
The Journal of clinical investigation
125
2661-76
2015
Show Abstract
In mammals, the outflow tract (OFT) of the developing heart septates into the base of the pulmonary artery and aorta to guide deoxygenated right ventricular blood into the lungs and oxygenated left ventricular blood into the systemic circulation. Accordingly, defective OFT septation is a life-threatening condition that can occur in both syndromic and nonsyndromic congenital heart disease. Even though studies of genetic mouse models have previously revealed a requirement for VEGF-A, the class 3 semaphorin SEMA3C, and their shared receptor neuropilin 1 (NRP1) in OFT development, the precise mechanism by which these proteins orchestrate OFT septation is not yet understood. Here, we have analyzed a complementary set of ligand-specific and tissue-specific mouse mutants to show that neural crest-derived SEMA3C activates NRP1 in the OFT endothelium. Explant assays combined with gene-expression studies and lineage tracing further demonstrated that this signaling pathway promotes an endothelial-to-mesenchymal transition that supplies cells to the endocardial cushions and repositions cardiac neural crest cells (NCCs) within the OFT, 2 processes that are essential for septal bridge formation. These findings elucidate a mechanism by which NCCs cooperate with endothelial cells in the developing OFT to enable the postnatal separation of the pulmonary and systemic circulation. | | | 26053665
 |
Loss of Foxm1 Results in Reduced Somatotrope Cell Number during Mouse Embryogenesis.
Calderon, MJ; Ploegman, AG; Bailey, B; Jung, DO; Navratil, AM; Ellsworth, BS
PloS one
10
e0128942
2015
Show Abstract
FOXM1, a member of the forkhead box transcription factor family, plays a key role in cell cycling progression by regulating the expression of critical G1/S and G2/M phase transition genes. In vivo studies reveal that Foxm1 null mice have a 91% lethality rate at e18.5 due to significant cardiovascular and hepatic hypoplasia. Thus, FOXM1 has emerged as a key protein regulating mitotic division and cell proliferation necessary for embryogenesis. In the current study, we assess the requirement for Foxm1 in the developing pituitary gland. We find that Foxm1 is expressed in the pituitary at embryonic days 10.5-e18.5 and localizes with markers for active cell proliferation (BrdU). Interestingly, direct analysis of Foxm1 null mice at various embryonic ages, reveals no difference in gross pituitary morphology or cell proliferation. We do observe a downward trend in overall pituitary cell number and a small reduction in pituitary size in e18.5 embryos suggesting there may be subtle changes in pituitary proliferation not detected with our proliferation makers. Consistent with this, Foxm1 null mice have reductions in both the somatotrope and gonadotrope cell populations. | | | 26075743
 |
Age-dependent decline in fin regenerative capacity in the short-lived fish Nothobranchius furzeri.
Wendler, S; Hartmann, N; Hoppe, B; Englert, C
Aging cell
14
857-66
2015
Show Abstract
The potential to regenerate declines with age in a wide range of organisms. A popular model system to study the mechanisms of regeneration is the fin of teleost fish, which has the ability to fully regrow upon amputation. Here, we used the short-lived killifish Nothobranchius furzeri to analyse the impact of aging on fin regeneration in more detail. We observed that young fish were able to nearly completely (98%) regenerate their amputated caudal fins within 4 weeks, whereas middle-aged fish reached 78%, old fish 57% and very old fish 46% of their original fin size. The difference in growth rate between young and old fish was already significant at 3 days post amputation (dpa) and increased with time. We therefore hypothesized that early events are crucial for the age-related differences in regenerative capacity. Indeed, we could observe a higher percentage of proliferating cells in early regenerating fin tissue of young fish compared with aged fish and larger fractions of apoptotic cells in aged fish. Furthermore, young fish showed peak upregulation of several genes involved in fgf and wnt/β-catenin signalling at an earlier time point than old fish. Our findings suggest that regenerative processes are initiated earlier and that regeneration overall is more efficient in younger fish. | | | 26121607
 |
Identification of Chemical Inhibitors of β-Catenin-Driven Liver Tumorigenesis in Zebrafish.
Evason, KJ; Francisco, MT; Juric, V; Balakrishnan, S; Lopez Pazmino, Mdel P; Gordan, JD; Kakar, S; Spitsbergen, J; Goga, A; Stainier, DY
PLoS genetics
11
e1005305
2015
Show Abstract
Hepatocellular carcinoma (HCC) is one of the most lethal human cancers. The search for targeted treatments has been hampered by the lack of relevant animal models for the genetically diverse subsets of HCC, including the 20-40% of HCCs that are defined by activating mutations in the gene encoding β-catenin. To address this chemotherapeutic challenge, we created and characterized transgenic zebrafish expressing hepatocyte-specific activated β-catenin. By 2 months post fertilization (mpf), 33% of transgenic zebrafish developed HCC in their livers, and 78% and 80% of transgenic zebrafish showed HCC at 6 and 12 mpf, respectively. As expected for a malignant process, transgenic zebrafish showed significantly decreased mean adult survival compared to non-transgenic control siblings. Using this novel transgenic model, we screened for druggable pathways that mediate β-catenin-induced liver growth and identified two c-Jun N-terminal kinase (JNK) inhibitors and two antidepressants (one tricyclic antidepressant, amitriptyline, and one selective serotonin reuptake inhibitor) that suppressed this phenotype. We further found that activated β-catenin was associated with JNK pathway hyperactivation in zebrafish and in human HCC. In zebrafish larvae, JNK inhibition decreased liver size specifically in the presence of activated β-catenin. The β-catenin-specific growth-inhibitory effect of targeting JNK was conserved in human liver cancer cells. Our other class of hits, antidepressants, has been used in patient treatment for decades, raising the exciting possibility that these drugs could potentially be repurposed for cancer treatment. In support of this proposal, we found that amitriptyline decreased tumor burden in a mouse HCC model. Our studies implicate JNK inhibitors and antidepressants as potential therapeutics for β-catenin-induced liver tumors. | | | 26134322
 |
Context-Dependent Functional Divergence of the Notch Ligands DLL1 and DLL4 In Vivo.
Preuße, K; Tveriakhina, L; Schuster-Gossler, K; Gaspar, C; Rosa, AI; Henrique, D; Gossler, A; Stauber, M
PLoS genetics
11
e1005328
2015
Show Abstract
Notch signalling is a fundamental pathway that shapes the developing embryo and sustains adult tissues by direct communication between ligand and receptor molecules on adjacent cells. Among the ligands are two Delta paralogues, DLL1 and DLL4, that are conserved in mammals and share a similar structure and sequence. They activate the Notch receptor partly in overlapping expression domains where they fulfil redundant functions in some processes (e.g. maintenance of the crypt cell progenitor pool). In other processes, however, they appear to act differently (e.g. maintenance of foetal arterial identity) raising the questions of how similar DLL1 and DLL4 really are and which mechanism causes the apparent context-dependent divergence. By analysing mice that conditionally overexpress DLL1 or DLL4 from the same genomic locus (Hprt) and mice that express DLL4 instead of DLL1 from the endogenous Dll1 locus (Dll1Dll4ki), we found functional differences that are tissue-specific: while DLL1 and DLL4 act redundantly during the maintenance of retinal progenitors, their function varies in the presomitic mesoderm (PSM) where somites form in a Notch-dependent process. In the anterior PSM, every cell expresses both Notch receptors and ligands, and DLL1 is the only activator of Notch while DLL4 is not endogenously expressed. Transgenic DLL4 cannot replace DLL1 during somitogenesis and in heterozygous Dll1Dll4ki/+ mice, the Dll1Dll4ki allele causes a dominant segmentation phenotype. Testing several aspects of the complex Notch signalling system in vitro, we found that both ligands have a similar trans-activation potential but that only DLL4 is an efficient cis-inhibitor of Notch signalling, causing a reduced net activation of Notch. These differential cis-inhibitory properties are likely to contribute to the functional divergence of DLL1 and DLL4. | | | 26114479
 |
Midostaurin preferentially attenuates proliferation of triple-negative breast cancer cell lines through inhibition of Aurora kinase family.
Kawai, M; Nakashima, A; Kamada, S; Kikkawa, U
Journal of biomedical science
22
48
2015
Show Abstract
Breast cancer is classified into three subtypes by the expression of biomarker receptors such as hormone receptors and human epidermal growth factor receptor 2. Triple-negative breast cancer (TNBC) expresses none of these receptors and has an aggressive phenotype with a poor prognosis, which is insensitive to the drugs that target the hormone receptors and human epidermal growth factor receptor 2. It is, thus, required to develop an effective therapeutic reagent to treat TNBC.The study using a panel of 19 breast cancer cell lines revealed that midostaurin, a multi-target protein kinase inhibitor, suppresses preferentially the growth of TNBC cells comparing with non-TNBC cells. Clustering analysis of the drug activity data for the panel of cancer cell lines predicted that midostaurin shares the target with Aurora kinase inhibitors. Following studies indicated that midostaurin attenuates the phosphorylation reaction mediated by Aurora kinase in the cells and directly inhibits this protein kinase in vitro, and that this reagent induces apoptosis accompanying accumulation of 4N and 8N DNA cells in TNBC cells.Midostaurin suppresses the proliferation of TNBC cells among the breast cancer cell lines presumably through the inhibition of the Aurora kinase family. The precise study of midostaurin on cell growth will contribute to the development of the drug for the treatment of TNBC. | | | 26141684
 |
TD-60 links RalA GTPase function to the CPC in mitosis.
Papini, D; Langemeyer, L; Abad, MA; Kerr, A; Samejima, I; Eyers, PA; Jeyaprakash, AA; Higgins, JM; Barr, FA; Earnshaw, WC
Nature communications
6
7678
2015
Show Abstract
TD-60 (also known as RCC2) is a highly conserved protein that structurally resembles the Ran guanine exchange factor (GEF) RCC1, but has not previously been shown to have GEF activity. TD-60 has a typical chromosomal passenger complex (CPC) distribution in mitotic cells, but associates with integrin complexes and is involved in cell motility during interphase. Here we show that TD-60 exhibits GEF activity, in vitro and in cells, for the small GTPase RalA. TD-60 or RalA depletion causes spindle abnormalities in prometaphase associated with abnormal centromeric accumulation of CPC components. TD-60 and RalA apparently work together to contribute to the regulation of kinetochore-microtubule interactions in early mitosis. Importantly, several mitotic phenotypes caused by TD-60 depletion are reverted by the expression of a GTP-locked mutant, RalA (Q72L). The demonstration that a small GTPase participates in the regulation of the CPC reveals a level of mitotic regulation not suspected in previous studies. | | | 26158537
 |
Shh Signaling through the Primary Cilium Modulates Rat Oligodendrocyte Differentiation.
Falcón-Urrutia, P; Carrasco, CM; Lois, P; Palma, V; Roth, AD
PloS one
10
e0133567
2015
Show Abstract
Primary Cilia (PC) are a very likely place for signal integration where multiple signaling pathways converge. Two major signaling pathways clearly shown to signal through the PC, Sonic Hedgehog (Shh) and PDGF-Rα, are particularly important for the proliferation and differentiation of oligodendrocytes, suggesting that their interaction occurs in or around this organelle. We identified PC in rat oligodendrocyte precursor cells (OPCs) and found that, while easily detectable in early OPCs, PC are lost as these cells progress to terminal differentiation. We confirmed the interaction between these pathways, as cyclopamine inhibition of Hedgehog function impairs both PDGF-mediated OPC proliferation and Shh-dependent cell branching. However, we failed to detect PDGF-Rα localization into the PC. Remarkably, ciliobrevin-mediated disruption of PC and reduction of OPC process extension was counteracted by recombinant Shh treatment, while PDGF had no effect. Therefore, while PDGF-Rα-dependent OPC proliferation and survival most probably does not initiate at the PC, still the integrity of this organelle and cilium-centered pathway is necessary for OPC survival and differentiation. | | | 26218245
 |