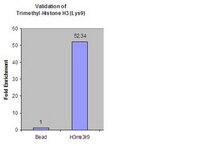
Independent Mechanisms Target SMCHD1 to Trimethylated Histone H3 Lysine 9-Modified Chromatin and the Inactive X Chromosome.
Brideau, NJ; Coker, H; Gendrel, AV; Siebert, CA; Bezstarosti, K; Demmers, J; Poot, RA; Nesterova, TB; Brockdorff, N
Molecular and cellular biology
35
4053-68
2015
Show Abstract
The chromosomal protein SMCHD1 plays an important role in epigenetic silencing at diverse loci, including the inactive X chromosome, imprinted genes, and the facioscapulohumeral muscular dystrophy locus. Although homology with canonical SMC family proteins suggests a role in chromosome organization, the mechanisms underlying SMCHD1 function and target site selection remain poorly understood. Here we show that SMCHD1 forms an active GHKL-ATPase homodimer, contrasting with canonical SMC complexes, which exist as tripartite ring structures. Electron microscopy analysis demonstrates that SMCHD1 homodimers structurally resemble prokaryotic condensins. We further show that the principal mechanism for chromatin loading of SMCHD1 involves an LRIF1-mediated interaction with HP1γ at trimethylated histone H3 lysine 9 (H3K9me3)-modified chromatin sites on the chromosome arms. A parallel pathway accounts for chromatin loading at a minority of sites, notably the inactive X chromosome. Together, our results provide key insights into SMCHD1 function and target site selection. | | 26391951
 |
miR-142-5p and miR-130a-3p are regulated by IL-4 and IL-13 and control profibrogenic macrophage program.
Su, S; Zhao, Q; He, C; Huang, D; Liu, J; Chen, F; Chen, J; Liao, JY; Cui, X; Zeng, Y; Yao, H; Su, F; Liu, Q; Jiang, S; Song, E
Nature communications
6
8523
2015
Show Abstract
Macrophages play a pivotal role in tissue fibrogenesis, which underlies the pathogenesis of many end-stage chronic inflammatory diseases. MicroRNAs are key regulators of immune cell functions, but their roles in macrophage's fibrogenesis have not been characterized. Here we show that IL-4 and IL-13 induce miR-142-5p and downregulate miR-130a-3p in macrophages; these changes sustain the profibrogenic effect of macrophages. In vitro, miR-142-5p mimic prolongs STAT6 phosphorylation by targeting its negative regulator, SOCS1. Blocking miR-130a relieves its inhibition of PPARγ, which coordinates STAT6 signalling. In vivo, inhibiting miR-142-5p and increasing miR-130a-3p expression with locked nucleic acid-modified oligonucleotides inhibits CCL4-induced liver fibrosis and bleomycin-induced lung fibrosis in mice. Furthermore, macrophages from the tissue samples of patients with liver cirrhosis and idiopathic pulmonary fibrosis display increased miR-142-5p and decreased miR-130a-3p expression. Therefore, miR-142-5p and miR-130a-3p regulate macrophage profibrogenic gene expression in chronic inflammation. | | 26436920
 |
Ligand-dependent corepressor contributes to transcriptional repression by C2H2 zinc-finger transcription factor ZBRK1 through association with KRAB-associated protein-1.
Calderon, MR; Verway, M; Benslama, RO; Birlea, M; Bouttier, M; Dimitrov, V; Mader, S; White, JH
Nucleic acids research
42
7012-27
2014
Show Abstract
We identified a novel interaction between ligand-dependent corepressor (LCoR) and the corepressor KRAB-associated protein-1 (KAP-1). The two form a complex with C2H2 zinc-finger transcription factor ZBRK1 on an intronic binding site in the growth arrest and DNA-damage-inducible α (GADD45A) gene and a novel site in the fibroblast growth factor 2 (FGF2) gene. Chromatin at both sites is enriched for histone methyltransferase SETDB1 and histone 3 lysine 9 trimethylation, a repressive epigenetic mark. Depletion of ZBRK1, KAP-1 or LCoR led to elevated GADD45A and FGF2 expression in malignant and non-malignant breast epithelial cells, and caused apoptotic death. Loss of viability could be rescued by simultaneous knockdowns of FGF2 and transcriptional coregulators or by blocking FGF2 function. FGF2 was not concurrently expressed with any of the transcriptional coregulators in breast malignancies, suggesting an inverse correlation between their expression patterns. We propose that ZBRK1, KAP-1 and LCoR form a transcriptional complex that silences gene expression, in particular FGF2, which maintains breast cell viability. Given the broad expression patterns of both LCoR and KAP-1 during development and in the adult, this complex may have several regulatory functions that extend beyond cell survival, mediated by interactions with ZBRK1 or other C2H2 zinc-finger proteins. | | 24829459
 |
Suv39h1 mediates AP-2α-dependent inhibition of C/EBPα expression during adipogenesis.
Zhang, ZC; Liu, Y; Li, SF; Guo, L; Zhao, Y; Qian, SW; Wen, B; Tang, QQ; Li, X
Molecular and cellular biology
34
2330-8
2014
Show Abstract
Previous studies have shown that CCAAT/enhancer-binding protein α (C/EBPα) plays a very important role during adipocyte terminal differentiation and that AP-2α (activator protein 2α) acts as a repressor to delay the expression of C/EBPα. However, the mechanisms by which AP-2α prevents the expression of C/EBPα are not fully understood. Here, we present evidence that Suv39h1, a histone H3 lysine 9 (H3K9)-specific trimethyltransferase, and G9a, a euchromatic methyltransferase, both interact with AP-2α and enhance AP-2α-mediated transcriptional repression of C/EBPα. Interestingly, we discovered that G9a mediates dimethylation of H3K9, thus providing the substrate, which is methylated by Suv39h1, to H3K9me3 on the C/EBPα promoter. The expression level of AP-2α was consistent with enrichment of H3K9me2 and H3K9me3 on the C/EBPα promoter in 3T3-L1 preadipocytes. Knockdown of Suv39h markedly increased C/EBPα expression and promoted adipogenesis. Conversely, ectopic expression of Suv39h1 delayed C/EBPα expression and impaired the accumulation of triglyceride, while simultaneous knockdown of AP-2α or G9a partially rescued this process. These findings indicate that Suv39h1 enhances AP-2α-mediated transcriptional repression of C/EBPα in an epigenetic manner and further inhibits adipocyte differentiation. | Western Blotting | 24732798
 |
Alterations of epigenetic signatures in hepatocyte nuclear factor 4α deficient mouse liver determined by improved ChIP-qPCR and (h)MeDIP-qPCR assays.
Zhang, Q; Lei, X; Lu, H
PloS one
9
e84925
2014
Show Abstract
Hepatocyte nuclear factor 4α (HNF4α) is a liver-enriched transcription factor essential for liver development and function. In hepatocytes, HNF4α regulates a large number of genes important for nutrient/xenobiotic metabolism and cell differentiation and proliferation. Currently, little is known about the epigenetic mechanism of gene regulation by HNF4α. In this study, the global and specific alterations at the selected gene loci of representative histone modifications and DNA methylations were investigated in Hnf4a-deficient female mouse livers using the improved MeDIP-, hMeDIP- and ChIP-qPCR assay. Hnf4a deficiency significantly increased hepatic total IPed DNA fragments for histone H3 lysine-4 dimethylation (H3K4me2), H3K4me3, H3K9me2, H3K27me3 and H3K4 acetylation, but not for H3K9me3, 5-methylcytosine,or 5-hydroxymethylcytosine. At specific gene loci, the relative enrichments of histone and DNA modifications were changed to different degree in Hnf4a-deficient mouse liver. Among the epigenetic signatures investigated, changes in H3K4me3 correlated the best with mRNA expression. Additionally, Hnf4a-deficient livers had increased mRNA expression of histone H1.2 and H3.3 as well as epigenetic modifiers Dnmt1, Tet3, Setd7, Kmt2c, Ehmt2, and Ezh2. In conclusion, the present study provides convenient improved (h)MeDIP- and ChIP-qPCR assays for epigenetic study. Hnf4a deficiency in young-adult mouse liver markedly alters histone methylation and acetylation, with fewer effects on DNA methylation and 5-hydroxymethylation. The underlying mechanism may be the induction of epigenetic enzymes responsible for the addition/removal of the epigenetic signatures, and/or the loss of HNF4α per se as a key coordinator for epigenetic modifiers. | | 24427299
 |
High levels of glucose induce metabolic memory in cardiomyocyte via epigenetic histone H3 lysine 9 methylation.
Xi-Yong Yu,Yong-Jian Geng,Jia-Liang Liang,Saidan Zhang,He-Ping Lei,Shi-Long Zhong,Qiu-Xiong Lin,Zhi-Xin Shan,Shu-Guang Lin,Yangxin Li
Molecular biology reports
39
2012
Show Abstract
Diabetic patients continue to develop inflammation and cardiovascular complication even after achieving glycemic control, suggesting a metabolic memory. Metabolic memory is a major challenge in the treatment of diabetic complication, and the mechanisms underlying metabolic memory are not clear. Recent studies suggest a link between chromatin histone methylation and metabolic memory. In this study, we tested whether histone 3 lysine-9 tri-methylation (H3K9me3), a key epigenetic chromatin marker, was involved in high glucose (HG)-induced inflammation and metabolic memory. Incubating cardiomyocyte cells in HG resulted in increased levels of inflammatory cytokine IL-6 mRNA when compared with myocytes incubated in normal culture media, whereas mannitol (osmotic control) has no effect. Chromatin immunoprecipitation (ChIP) assays showed that H3K9me3 levels were significantly decreased at the promoters of IL-6. Immunoblotting demonstrated that protein levels of the H3K9me3 methyltransferase, Suv39h1, were also reduced after HG treatment. HG-induced apoptosis, mitochondrial dysfunction and cytochrome-c release were reversible. However, the effects of HG on the expression of IL-6 and the levels of H3K9me3 were irreversible after the removal of HG from the culture. These results suggest that HG-induced sustained inflammatory phenotype and epigenetic histone modification, rather than HG-induced mitochondrial dysfunction and apoptosis, are main mechanisms responsible for metabolic memory. In conclusion, our data demonstrate that HG increases expression of inflammatory cytokine and decreases the levels of histone-3 methylation at the cytokine promoter, and suggest that modulating histone 3 methylation and inflammatory cytokine expression may be a useful strategy to prevent metabolic memory and cardiomyopathy in diabetic patients. | | 22707199
 |
Compensatory functions of histone deacetylase 1 (HDAC1) and HDAC2 regulate transcription and apoptosis during mouse oocyte development.
Ma, P; Pan, H; Montgomery, RL; Olson, EN; Schultz, RM
Proceedings of the National Academy of Sciences of the United States of America
109
E481-9
2012
Show Abstract
Dramatic changes in chromatin structure and histone modification occur during oocyte growth, as well as a global cessation of transcription. The role of histone modifications in these processes is poorly understood. We report the effect of conditionally deleting Hdac1 and Hdac2 on oocyte development. Deleting either gene has little or no effect on oocyte development, whereas deleting both genes results in follicle development arrest at the secondary follicle stage. This developmental arrest is accompanied by substantial perturbation of the transcriptome and a global reduction in transcription even though histone acetylation is markedly increased. There is no apparent change in histone repressive marks, but there is a pronounced decrease in histone H3K4 methylation, an activating mark. The decrease in H3K4 methylation is likely a result of increased expression of Kdm5b because RNAi-mediated targeting of Kdm5b in double-mutant oocytes results in an increase in H3K4 methylation. An increase in TRP53 acetylation also occurs in mutant oocytes and may contribute to the observed increased incidence of apoptosis. Taken together, these results suggest seminal roles of acetylation of histone and nonhistone proteins in oocyte development. | Immunofluorescence | 22223663
 |
p53-mediated heterochromatin reorganization regulates its cell fate decisions.
Mungamuri, SK; Benson, EK; Wang, S; Gu, W; Lee, SW; Aaronson, SA
Nature structural & molecular biology
19
478-84, S1
2012
Show Abstract
p53 is a major sensor of cellular stresses, and its activation influences cell fate decisions. We identified SUV39H1, a histone code 'writer' responsible for the histone H3 Lys9 trimethylation (H3K9me3) mark for 'closed' chromatin conformation, as a target of p53 repression. SUV39H1 downregulation was mediated transcriptionally by p21 and post-translationally by MDM2. The H3K9me3 repression mark was found to be associated with promoters of representative p53 target genes and was decreased upon p53 activation. Overexpression of SUV39H1 maintained higher levels of the H3K9me3 mark on these promoters and was associated with decreased p53 promoter occupancy and decreased transcriptional induction in response to p53. Conversely, SUV39H1 pre-silencing decreased H3K9me3 levels on these promoters and enhanced the p53 apoptotic response. These findings uncover a new layer of p53-mediated chromatin regulation through modulation of histone methylation at p53 target promoters. | Western Blotting | 22466965
 |
Epigenomics: maternal high-fat diet exposure in utero disrupts peripheral circadian gene expression in nonhuman primates.
Suter, M; Bocock, P; Showalter, L; Hu, M; Shope, C; McKnight, R; Grove, K; Lane, R; Aagaard-Tillery, K
FASEB journal : official publication of the Federation of American Societies for Experimental Biology
25
714-26
2011
Show Abstract
The effect of in utero exposure to a maternal high-fat diet on the peripheral circadian system of the fetus is unknown. Using mRNA copy number analysis, we report that the components of the peripheral circadian machinery are transcribed in the nonhuman primate fetal liver in an intact phase-antiphase fashion and that Npas2, a paralog of the Clock transcription factor, serves as the rate-limiting transcript by virtue of its relative low abundance (10- to 1000-fold lower). We show that exposure to a maternal high-fat diet in utero significantly alters the expression of fetal hepatic Npas2 (up to 7.1-fold, Pless than 0.001) compared with that in control diet-exposed animals and is reversible in fetal offspring from obese dams reversed to a control diet (1.3-fold, Pgreater than 0.05). Although the Npas2 promoter remains largely unmethylated, differential Npas2 promoter occupancy of acetylation of fetal histone H3 at lysine 14 (H3K14ac) occurs in response to maternal high-fat diet exposure compared with control diet-exposed animals. Furthermore, we find that disruption of Npas2 is consistent with high-fat diet exposure in juvenile animals, regardless of in utero diet exposure. In summary, the data suggest that peripheral Npas2 expression is uniquely vulnerable to diet exposure. | | 21097519
 |
Cooperative and antagonistic contributions of two heterochromatin proteins to transcriptional regulation of the Drosophila sex determination decision.
Li, H; Rodriguez, J; Yoo, Y; Shareef, MM; Badugu, R; Horabin, JI; Kellum, R
PLoS genetics
7
e1002122
2011
Show Abstract
Eukaryotic nuclei contain regions of differentially staining chromatin (heterochromatin), which remain condensed throughout the cell cycle and are largely transcriptionally silent. RNAi knockdown of the highly conserved heterochromatin protein HP1 in Drosophila was previously shown to preferentially reduce male viability. Here we report a similar phenotype for the telomeric partner of HP1, HOAP, and roles for both proteins in regulating the Drosophila sex determination pathway. Specifically, these proteins regulate the critical decision in this pathway, firing of the establishment promoter of the masterswitch gene, Sex-lethal (Sxl). Female-specific activation of this promoter, Sxl(Pe), is essential to females, as it provides SXL protein to initiate the productive female-specific splicing of later Sxl transcripts, which are transcribed from the maintenance promoter (Sxl(Pm)) in both sexes. HOAP mutants show inappropriate Sxl(Pe) firing in males and the concomitant inappropriate splicing of Sxl(Pm)-derived transcripts, while females show premature firing of Sxl(Pe). HP1 mutants, by contrast, display Sxl(Pm) splicing defects in both sexes. Chromatin immunoprecipitation assays show both proteins are associated with Sxl(Pe) sequences. In embryos from HP1 mutant mothers and Sxl mutant fathers, female viability and RNA polymerase II recruitment to Sxl(Pe) are severely compromised. Our genetic and biochemical assays indicate a repressing activity for HOAP and both activating and repressing roles for HP1 at Sxl(Pe). | | 21695246
 |