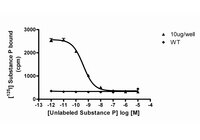
The tachykinin peptide family.
Severini, Cinzia, et al.
Pharmacol. Rev., 54: 285-322 (2002)
2002
Show Abstract
The tachykinin peptide family certainly represents one of the largest peptide families described in the animal organism. So far, more than 40 tachykinins have been isolated from invertebrate (insects, worms, and molluscs), protochordate, and vertebrate (skin, gastrointestinal tract, peripheral and central nervous system) tissues. Substance P (SP), first identified by bioassay as early as 1931 but sequenced only in 1971, several years after the elucidation of the structure of eledoisin from molluscan tissues and of physalaemin from amphibian skin, may be considered as a prototype of the tachykinins. Hitherto, as many as 19 tachykinins have been isolated from amphibian integument, and eight additional peptides have been isolated from amphibian gut and brain. Counterparts of skin tachykinins in mammalian tissues are SP, neurokinin A, and neurokinin B. Three main receptor subtypes for the tachykinins have been identified (NK1, NK2, and NK3), but their number is probably destined to increase. It is obvious that the peripheral and central effects of the tachykinins may substantially vary depending on the activation of different receptor subtypes. Matters are further complicated by the frequent capacity of the single tachykinins to bind, although with different affinity, to more receptors. It has been recognized that tachykinins have a variety of effects in physiological and pathological conditions, and there is evidence suggesting intrinsic neuroprotective and neurodegenerative properties of these neuropeptides. This review provides an update on the current body of knowledge regarding tachykinin occurrence and distribution in the animal kingdom, from the lowest invertebrates to man, and the physiological and pharmacological actions of tachykinins outlining the pregnant importance of this large peptide family. | 12037144
 |
NK1 (substance P) receptor antagonists--why are they not analgesic in humans?
Hill, R
Trends Pharmacol. Sci., 21: 244-6 (2000)
2000
Show Abstract
Tachykinin NK1 receptor antagonists have failed to exhibit efficacy in clinical trials of a variety of clinical pain states. By contrast, in preclinical studies in animals NK1 receptor antagonists have been shown to attenuate nociceptive responses sensitized by inflammation or nerve damage, although they exhibit little effect on baseline nociception. Other agents with this profile of activity in animal tests, typically nonsteroidal anti-inflammatory drugs (NSAIDs), are analgesic in humans. Thus, NK1 receptor antagonists appear able to block behavioural responses to noxious and other stressful sensory stimuli at a level detectable in animal tests but fail to provide the level of sensory blockade required to produce clinical analgesia in humans. | 10871891
 |
The tachykinin NK1 receptor in the brain: pharmacology and putative functions.
Saria, A
Eur. J. Pharmacol., 375: 51-60 (1999)
1999
Show Abstract
After its discovery in 1931, substance P (SP) remained the only mammalian member of the family of tachykinin peptides for several decades. Tachykinins thus refer to peptides sharing the common C-terminal amino acid sequence Phe-X-Gly-Leu-Met x NH2. In recent years the family of mammalian tachykinins has grown with the isolation of two novel peptides from bovine and porcine central nervous system (CNS), neurokinin A and neurokinin B. In parallel with the identification of multiple endogenous tachykinins several classes of tachykinin receptors were discovered. The receptors described so far are named tachykinin NK1 receptor, tachykinin NK2 receptor and tachykinin NK1 receptor, respectively. The present review focuses on the pharmacology and putative function of tachykinin NK1 receptors in brain. The natural ligand with the highest affinity for the tachykinin NK1 receptor is SP itself. The C-terminal sequence is essential for activity, the minimum length of a fragment with reasonable affinity for the tachykinin NK1 receptor is the C-terminal hexapeptide. A rapid advance of knowledge was caused by development of non-peptidic tachykinin NK1 receptor antagonists. This area is under rapid development and a variety of different chemical classes of compounds are involved. Species-dependent affinities of tachykinin NK1 receptor antagonists reveal two clusters of compounds, targeting the tachykinin NK1 receptor subtype found in guinea pig, human or ferret or the one in rat or mouse, respectively. The most recently developed compounds are highly selective, enter the brain and are orally bioavailable. Distinct behavioural effects in experimental animals suggest the involvement of tachykinin NK1 receptors in nociceptive transmission, basal ganglia function or anxiety and depression. Recent clinical trials in man showed that tachykinin NK1 receptor antagonists are effective in treating depression and chemotherapy-induced emesis. Therefore, it is well possible that tachykinin NK1 receptor antagonists will be clinically used for treatment of specific CNS disorders within a short period of time. | 10443564
 |