365250 Sigma-AldrichGö 6976 - CAS 136194-77-9 - Calbiochem
Gö 6976, CAS 136194-77-9, is a cell-permeable, reversible, and ATP-competitive inhibitor of PKC (IC₅₀ = 7.9 nM for rat brain). Exhibits selectively for PKCα (IC₅₀ = 2.3 nM) and βI (IC₅₀ = 6.2 nM).
More>> Gö 6976, CAS 136194-77-9, is a cell-permeable, reversible, and ATP-competitive inhibitor of PKC (IC₅₀ = 7.9 nM for rat brain). Exhibits selectively for PKCα (IC₅₀ = 2.3 nM) and βI (IC₅₀ = 6.2 nM). Less<<Synonyms: Go 6976, 12-(2-Cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)-carbazole
Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| CAS # | Empirical Formula |
|---|---|
| 136194-77-9 | C₂₄H₁₈N₄O |
Pricing & Availability
| Catalog Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 365250-1MG |
|
Plastic ampoule | 1 mg |
|
— | |
| 365250-500UG |
|
Plastic ampoule | 500 μg |
|
— |
| Description | |
|---|---|
| Overview | A cell-permeable, reversible, and ATP-competitive inhibitor of protein kinase C (PKC; IC50 = 7.9 nM for rat brain). Selectively inhibits Ca2+-dependent PKC α-isozyme (IC50 = 2.3 nM) and PKCβI (IC50 = 6.2 nM). Does not affect the kinase activity of the Ca2+-independent PKC δ-, ε-, and ζ-isozymes even at micromolar levels. Reported to inhibit PKCµ at higher concentrations (IC50 = 20 nM). A 500 µg/ml solution of Gö 6976 (Cat. No. 365253) in anhydrous DMSO is also available. |
| Catalogue Number | 365250 |
| Brand Family | Calbiochem® |
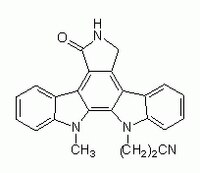
| Synonyms | Go 6976, 12-(2-Cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)-carbazole |
| Product Information | |
|---|---|
| CAS number | 136194-77-9 |
| ATP Competitive | Y |
| Form | Off-white solid |
| Hill Formula | C₂₄H₁₈N₄O |
| Chemical formula | C₂₄H₁₈N₄O |
| Reversible | Y |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | PKC |
| Primary Target IC<sub>50</sub> | 7.9 nM against rat brain PKC; 2.3 nM, 6.2 nM against Ca2+-dependent PKC α-isozyme and PKCβI, respectively |
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| 365250-1MG | 04055977192018 |
| 365250-500UG | 07790788049614 |
Documentation
Gö 6976 - CAS 136194-77-9 - Calbiochem SDS
| Title |
|---|
Gö 6976 - CAS 136194-77-9 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 365250 |
References
| Reference overview |
|---|
| Gschwendt, M., et al. 1996. FEBS Lett. 392, 77. Wenzel-Seifert, K., et al. 1994. Biochem. Biophys. Res. Commun. 200, 1536. Martiny-Baron, G.M., et al. 1993. J. Biol. Chem. 268, 9194. Qatsha, K.A., et al. 1993. Proc. Natl. Acad. Sci. USA 90, 4674. |
Brochure
| Title |
|---|
| PKC Pathway Poster PDF ( 676 KB ) |
Citations
| Title | |
|---|---|
|
|