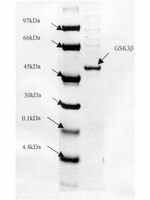
The alpha-isoform of glycogen synthase kinase-3 from rabbit skeletal muscle is inactivated by p70 S6 kinase or MAP kinase-activated protein kinase-1 in vitro.
Sutherland, C and Cohen, P
FEBS Lett., 338: 37-42 (1994)
1994
Show Abstract
The alpha-isoform of glycogen synthase kinase-3 (GSK3 alpha) was inactivated by 80% towards a synthetic peptide substrate upon incubation with Mg-ATP and either MAP kinase-activated protein (MAPKAP) kinase-1 or p70 S6 kinase. Inactivation by either kinase resulted from the phosphorylation of Ser-21 and was reversed by treatment with protein phosphatase 2A1. Phosphorylation also decreased GSK3 alpha activity towards glycogen synthase, inhibitor-2 and c-jun. The specificity of GSK3 alpha was similar to GSK3 beta, but with the synthetic peptide substrate heparin stimulated the dephosphorylated form of GSK3 alpha (6-fold) more than GSK3 beta (1.8-fold). After phosphorylation, both isoforms were stimulated 15-20-fold by heparin. | | 8307153
 |
Regulation and functions of the glycogen synthase kinase-3 subfamily.
Woodgett, J R
Semin. Cancer Biol., 5: 269-75 (1994)
1994
Show Abstract
Glycogen synthase kinase-3 is a 'black sheep' among protein kinases. Although its name suggests a primary function in intermediary metabolism, its scope is far more diverse as indicated by both genetic and biochemical evidence. Over the past five years the enzyme has emerged as an important component of transduction pathways ultimately regulating cell fate and differentiation. The molecular mechanisms underlying these functions are only just being unraveled but point to a novel mode of cellular regulation conserved in all eukaryotes. | | 7803763
 |