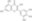476275 Sigma-AldrichMyricetin - CAS 529-44-2 - Calbiochem
A cell-permeable flavanoid that displays anti-inflammatory, anti-diabetic and anti-cancer properties.
More>> A cell-permeable flavanoid that displays anti-inflammatory, anti-diabetic and anti-cancer properties. Less<<Synonyms: 3,3ʹ,4ʹ,5,5ʹ,7-Hexahydroxyflavone, Hsp70 Inhibitor II
Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| CAS # | Empirical Formula |
|---|---|
| 529-44-2 | C₁₅H₁₀O₈ |
Pricing & Availability
| Catalog Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 476275-25MG |
|
Plastic ampoule | 25 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 529-44-2 |
| Form | Yellowish brown solid |
| Hill Formula | C₁₅H₁₀O₈ |
| Chemical formula | C₁₅H₁₀O₈ |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥98% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| 476275-25MG | 04055977184471 |
Documentation
Myricetin - CAS 529-44-2 - Calbiochem SDS
| Title |
|---|
Myricetin - CAS 529-44-2 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 476275 |
References
| Reference overview |
|---|
| Lee, K.W., et al. 2007. Carcinogenesis 28, 1918. Holder, S., et al. 2007. Mol. Cancer Ther. 6, 163. Strobel, P., et al. 2005. Biochem. J. 386, 471. Ko, C.H., et al. 2005. Mol. Cancer Ther. 4, 281. Ko, C.H., et al. 2005. Biochem. Pharmacol. 69, 913. Ko, W.C., et al. 2004. Biochem. Pharmacol. 68, 2087. Walker, E.H., et al. 2000. Mol. Cell 6, 909. Agullo, G., et al. 1997. Biochem. Pharmacol. 53, 1649. Hagiwara, M., et al. 1988. Biochem. Pharmacol. 37, 2987. |