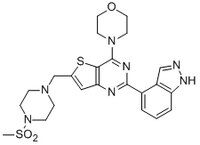
509226 Sigma-AldrichPI 3-K Inhibitor XXI, GDC-0941 - CAS 957054-30-7 - Calbiochem
A cell-permeable, potent, ATP-competitive inhibitor of class IA (IC₅₀ = 3 nM/p110α, 33 nM/p110&beta, 3 nM/p110δ) and IB PI 3-kinases (IC₅₀ = 75 nM/p110γ).
More>> A cell-permeable, potent, ATP-competitive inhibitor of class IA (IC₅₀ = 3 nM/p110α, 33 nM/p110&beta, 3 nM/p110δ) and IB PI 3-kinases (IC₅₀ = 75 nM/p110γ). Less<<Synonyms: GDC0941, 2-(1H-Indazol-4-yl)-6-(4-methylsulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2- d]pyrimidine, 4-(2-(1H-Indazol-4-yl)-6-((4-(methylsulfonyl)piperazin-1-yl)methyl)thieno[3,2-d]pyrimidin-4-yl)morpholine
Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| CAS # | Empirical Formula |
|---|---|
| 957054-30-7 | C₂₃H₂₇N₇O₃S₂ |
Pricing & Availability
| Catalog Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 5.09226.0001 |
|
Glass bottle | 10 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 957054-30-7 |
| Form | White solid |
| Hill Formula | C₂₃H₂₇N₇O₃S₂ |
| Chemical formula | C₂₃H₂₇N₇O₃S₂ |
| Reversible | Y |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | PI 3-K |
| Purity | ≥99% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| 5.09226.0001 | 04055977241693 |
Documentation
PI 3-K Inhibitor XXI, GDC-0941 - CAS 957054-30-7 - Calbiochem SDS
| Title |
|---|
References
| Reference overview |
|---|
| Tabe, Y., et al. 2014. Acta Haematol. 131, 59. Ware, J.A., et al. 2013. Mol. Pharm. 10, 4047. Junttila,.T., et al. 2009. Cancer Cell 15, 429. Raynaud, F.I., et al. 2009. Mol Cancer Ther. 8, 1725. Folkes, A. J., et al. 2008. J. Med. Chem. 51, 5522. |