ECM558 Sigma-AldrichQCM™ Tumor Cell Transendothelial Migration Assay (Colorimetric, 24 Assays)
This QCM Tumor Cell Transendothelial Cell Migration Assay -Colorimetric provides an efficient model to analyze the ability of tumor cells to invade the endothelium.
More>> This QCM Tumor Cell Transendothelial Cell Migration Assay -Colorimetric provides an efficient model to analyze the ability of tumor cells to invade the endothelium. Less<<Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| Detection Methods |
|---|
| Colorimetric |
| Description | |
|---|---|
| Catalogue Number | ECM558 |
| Trade Name |
|
| Description | QCM™ Tumor Cell Transendothelial Migration Assay (Colorimetric, 24 Assays) |
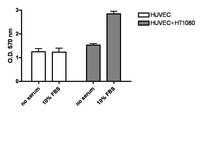
| Overview | Also available: Cell Comb™ Scratch Assay! Get biochemical data from a scratch assay! Click Here Introduction A quantitative assay for tumor transendothelial migration has been described using a modified Boyden chamber system, (Okada, 1994; Li, 1999; Laferriere, 2001). The Boyden Chamber system is a two-chamber system with a porous membrane providing an interface between these two chambers. Endothelial cells are cultured on top of the porous membrane that is coated with an extracelluar matrix (ECM) protein. A tumor cell suspension is added above the endothelial monolayer. The invasion of tumor cells across the endothelium is determined by measuring the number of cells that migrate to the lower chamber. Millipore's QCM Tumor Cell Transendothelial Cell Migration Assay - Colorimetric provides an efficient model to analyze the ability of tumor cells to invade the endothelium. The assay is designed with an 8 µm pore size cell culture insert, appropriate for most cancer cell lines. The upper side of the cell culture insert is coated with fibronectin to support the optimal attachment and growth of endothelial cells. The assay allows investigators to compare the invasiveness of a variety of tumor cell lines, and to evaluate the effects of various factors influencing the process. Precoated cell culture inserts are provided in the Millipore QCM Tumor Cell Transendothelial Cell Migration Assay to significantly reduce assay time. Additionally, the assay allows quantitative analysis of tumor cell migration. Following incubation of tumor cells with the endothelial cell layer, invasive tumor cells are stained and quantified. In a departure from traditional Boyden methodology, stain is eluted with extraction buffer, transferred to a microplate, and measured spectrophotometrically. (Prior to elution, the investigator has the option of counting cells individually, if desired.) Spectrophotometric absorbance correlates with cell migration. |
| Materials Required but Not Delivered | 1. Endothelial cells (for example: HUVECs), and cell culture medium (for example: EGM-2) 2. Invasive tumor cell lines and cell culture medium 3. Harvesting buffer: Millipore's cell detachment solution, Accutase™ (Cat. No. SCR005), EDTA or trypsin-based cell detachment buffer, or other cell detachment formulations as optimized by individual investigators can also be used 4. Serum-free medium, such as DMEM, MEM, etc. containing 0.5% BSA 5. Sterile PBS or HBSS to wash cells 6. Distilled water 7. (Optional) Chemoattractant or pharmacological agent for addition to culture medium 8. Low speed centrifuge and tubes for cell harvesting 9. CO2 incubator appropriate for subject cells 10. Hemocytometer or other means of counting cells 11. Trypan blue or equivalent viability stain 12. Microplate reader (540-570 nm detection) or spectrophotometer 13. (Optional) Graduated ocular (calibrated), or automated method for counting stained cells on a membrane |
| Background Information | Tumor metastasis is a multistep cascade. In order for tumor cells to migrate from a primary tumor mass to distant locations, they must invade through the basal membrane and into blood vessels (intravasation), circulate in the blood stream, survive during transport, then migrate out of a blood vessel (extravasation) to establish micrometastases. The penetration of circulating tumor cells into the endothelium is a crucial step for tumor metastasis. |
| References |
|---|
| Product Information | |
|---|---|
| Components |
|
| Detection method | Colorimetric |
| Quality Level | MQ100 |
| Biological Information |
|---|
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Storage and Shipping Information | |
|---|---|
| Storage Conditions | TNFα should be stored at -20°C. All other components should be store at 2° to 8°C. Please refer to kit label for expiration date. |
| Packaging Information | |
|---|---|
| Material Size | 1 kit |
| Material Package | 24 Assays |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| ECM558 | 04053252014758 |
Documentation
QCM™ Tumor Cell Transendothelial Migration Assay (Colorimetric, 24 Assays) SDS
| Title |
|---|
References
| Reference overview | Pub Med ID |
|---|---|
| Transendothelial migration of colon carcinoma cells requires expression of E-selectin by endothelial cells and activation of stress-activated protein kinase-2 (SAPK2/p38) in the tumor cells. Laferriere, J, et al. J. Biol. Chem., 276: 33762-72 (2001) 2001 Show Abstract | 11448946
 |
| A modified Boyden chamber assay for tumor cell transendothelial migration in vitro. Li, Y H and Zhu, C Clin. Exp. Metastasis, 17: 423-9 (1999) 1999 | 10651309
 |
| A novel in vitro assay system for transendothelial tumor cell invasion: significance of E-selectin and alpha 3 integrin in the transendothelial invasion by HT1080 fibrosarcoma cells. Okada, T, et al. Clin. Exp. Metastasis, 12: 305-14 (1994) 1994 | 7518760
 |