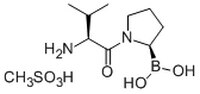
531465 Sigma-AldrichVal-boroPro - CAS 150080-09-4 - Calbiochem
An orally available, potent, reversible, and transition state analog inhibitor of DPP-IV (IC₅₀ = 26 nM)
More>> An orally available, potent, reversible, and transition state analog inhibitor of DPP-IV (IC₅₀ = 26 nM) Less<<Synonyms: H₂N-(S)-Val-(R)-boroPro-OH, CH₃SO₃H, H-(S)-Val-(R)-boroPro-OH, CH₃SO₃H, Valinyl-L-boroproline, CH₃SO₃H, DPP Inhibitor, PT-100, Talabostat
Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| CAS # | Empirical Formula |
|---|---|
| 150080-09-4 | C₁₀H₂₃BN₂O₆S |
Pricing & Availability
| Catalog Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 5.31465.0001 |
|
Glass bottle | 10 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 150080-09-4 |
| Form | Light beige solid |
| Hill Formula | C₁₀H₂₃BN₂O₆S |
| Chemical formula | C₁₀H₂₃BN₂O₆S |
| Hygroscopic | Hygroscopic |
| Reversible | Y |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | DPP-IV, DPP8 and DPP9 |
| Primary Target IC<sub>50</sub> | 15 nM for DPP-II |
| Primary Target K<sub>i</sub> | 180 pM |
| Secondary target | QPP, PEP and FAP |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| 5.31465.0001 | 04055977260328 |
Documentation
Val-boroPro - CAS 150080-09-4 - Calbiochem SDS
| Title |
|---|
Val-boroPro - CAS 150080-09-4 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 531465 |
References
| Reference overview |
|---|
| Poplawski, S.E., et al. 2011, J. Med. Chem. 54, 2022. Connolly, B.A., et al. 2008. J. Med. Chem. 51, 6005. Lankas, G.R., et al. 2005. Diabetes 54, 2988. Cheng, J.D., et al. 2005. Mol. Cancer Ther. 4, 351. Adams, S., et al. 2004. Cancer Res. 64, 5471. Jones, B., et al. 2003. Blood 102, 1641. Coutts, S.J., et al. 1996. J. Med. Chem. 39, 2087. Snow, R.J., et al. 1994. J. Am. Chem. Soc. 116, 10860. Kelly, T.A., et al. 1993. J. Am. Chem. Soc. 115, 12637. |