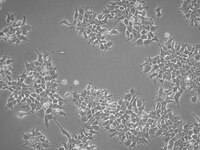
SCC295 Sigma-AldrichhERG1a/1b Inducible HEK293 Cell Line
The heteromeric hERG1a/1b Inducible HEK293 cell line, in which hERG1b protein expression is tightly controlled by doxycyclin dose and treatment time, was derived from a homomeric hERG 1a expressing HEK293 cell line
More>> The heteromeric hERG1a/1b Inducible HEK293 cell line, in which hERG1b protein expression is tightly controlled by doxycyclin dose and treatment time, was derived from a homomeric hERG 1a expressing HEK293 cell line Less<<Recommended Products
Overview
| Replacement Information |
|---|
| References |
|---|
| Product Information |
|---|
| Biological Information | |
|---|---|
| Cell Line Type |
|
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements | |
|---|---|
| Quality Assurance | • Each vial contains ≥ 1X10⁶ viable cells. • Cells are tested negative for infectious diseases by a Human Essential CLEAR panel by Charles River Animal Diagnostic Services. • Cells are verified to be of human origin and negative for inter-species contamination from mouse, rat, chinese hamster, Golden Syrian hamster, and Non-human Primate (NHP) as assessed by a Contamination Clear panel by Charles River Animal Diagnostic Services • Cells are negative for mycoplasma contamination. |
| Usage Statement |
|
| Packaging Information | |
|---|---|
| Material Size | ≥1X10⁶ cells/vial |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| SCC295 | 04065265944564 |
Documentation
Data Sheet
| Title |
|---|
| Data Sheet-SCC295 |