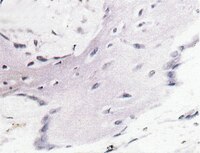
A Site-Specific Integrated Col2.3GFP Reporter Identifies Osteoblasts Within Mineralized Tissue Formed In Vivo by Human Embryonic Stem Cells.
Xin, X; Jiang, X; Wang, L; Stover, ML; Zhan, S; Huang, J; Goldberg, AJ; Liu, Y; Kuhn, L; Reichenberger, EJ; Rowe, DW; Lichtler, AC
Stem cells translational medicine
3
1125-37
2014
Show Abstract
The use of human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) for study and treatment of bone diseases or traumatic bone injuries requires efficient protocols to differentiate hESCs/iPSCs into cells with osteogenic potential and the ability to isolate differentiated osteoblasts for analysis. We have used zinc finger nuclease technology to deliver a construct containing the Col2.3 promoter driving GFPemerald to the AAVS1 site (referred to as a "safe harbor" site), in human embryonic stem cells (H9Zn2.3GFP), with the goal of marking the cells that have become differentiated osteoblasts. In teratomas formed using these cells, we identified green fluorescent protein (GFP)-positive cells specifically associated with in vivo bone formation. We also differentiated the cells into a mesenchymal stem cell population with osteogenic potential and implanted them into a mouse calvarial defect model. We observed GFP-positive cells associated with alizarin complexone-labeled newly formed bone surfaces. The cells were alkaline phosphatase-positive, and immunohistochemistry with human specific bone sialoprotein (BSP) antibody indicates that the GFP-positive cells are also associated with the human BSP-containing matrix, demonstrating that the Col2.3GFP construct marks cells in the osteoblast lineage. Single-cell cloning generated a 100% Col2.3GFP-positive cell population, as demonstrated by fluorescence in situ hybridization using a GFP probe. The karyotype was normal, and pluripotency was demonstrated by Tra1-60 immunostaining, pluripotent low density reverse transcription-polymerase chain reaction array and embryoid body formation. These cells will be useful to develop optimal osteogenic differentiation protocols and to isolate osteoblasts from normal and diseased iPSCs for analysis. | 25122686
 |
Developmental-like bone regeneration by human embryonic stem cell-derived mesenchymal cells.
Kuhn, LT; Liu, Y; Boyd, NL; Dennis, JE; Jiang, X; Xin, X; Charles, LF; Wang, L; Aguila, HL; Rowe, DW; Lichtler, AC; Goldberg, AJ
Tissue engineering. Part A
20
365-77
2014
Show Abstract
The in vivo osteogenesis potential of mesenchymal-like cells derived from human embryonic stem cells (hESC-MCs) was evaluated in vivo by implantation on collagen/hydroxyapatite scaffolds into calvarial defects in immunodeficient mice. This study is novel because no osteogenic or chondrogenic differentiation protocols were applied to the cells prior to implantation. After 6 weeks, X-ray, microCT, and histological analysis showed that the hESC-MCs had consistently formed a highly vascularized new bone that bridged the bone defect and seamlessly integrated with host bone. The implanted hESC-MCs differentiated in situ to functional hypertrophic chondrocytes, osteoblasts, and osteocytes forming new bone tissue via an endochondral ossification pathway. Evidence for the direct participation of the human cells in bone morphogenesis was verified by two separate assays: with Alu and by human mitochondrial antigen positive staining in conjunction with co-localized expression of human bone sialoprotein in histologically verified regions of new bone. The large volume of new bone in a calvarial defect and the direct participation of the hESC-MCs far exceeds that of previous studies and that of the control adult hMSCs. This study represents a key step forward for bone tissue engineering because of the large volume, vascularity, and reproducibility of new bone formation and the discovery that it is advantageous to not over-commit these progenitor cells to a particular lineage prior to implantation. The hESC-MCs were able to recapitulate the mesenchymal developmental pathway and were able to repair the bone defect semi-autonomously without preimplantation differentiation to osteo- or chondroprogenitors. | 23952622
 |
The effect of Cu(II)-loaded brushite scaffolds on growth and activity of osteoblastic cells.
Andrea Ewald,Christine Käppel,Elke Vorndran,Claus Moseke,Michael Gelinsky,Uwe Gbureck
Journal of biomedical materials research. Part A
100
2012
Show Abstract
Bone substitute materials such as calcium phosphate cements (CPC) are frequently used as growth factor carriers for the stimulation of osteoblast-formation around an implant. However, biological modification based on delicate protein factors like extracellular matrix proteins or growth factors is subject to a number of shortcomings like the need for storage below room temperature and cost of production. The aim of this study was to investigate ionic modification as an alternative bioinorganic route for implant modification. Although it is known that Cu(II) plays a role in angiogenesis and bone formation, not all involved processes are well understood yet. In this study the in vitro effect of Cu(II) on growth and activity of osteoblastic cells seeded on brushite (CaHPO(4) · 2 H(2) O) scaffolds as well as on glass discs was investigated. The results show that Cu(II) enhances cell activity and proliferation of osteoblastic cells on CPC and furthermore affects the expression of several bone specific proteins such as bone sialo protein or osteocalcin. Therefore, the modification of CPC with Cu(II) may offer a promising alternative to protein based modification to stimulate cellular activity for an improved bone healing. © 2012 Wiley Periodicals, Inc. J Biomed Mater Res Part A: 100A: 2392-2400, 2012. | 22528604
 |
Identification and characterization of the human leiomyoma side population as putative tumor-initiating cells.
Aymara Mas,Irene Cervelló,Claudia Gil-Sanchis,Amparo Faus,Jaime Ferro,Antonio Pellicer,Carlos Simón
Fertility and sterility
98
2012
Show Abstract
To isolate and characterize human leiomyoma stem cells by the side population (SP) method. | 22633281
 |
Reconstruction of endometrium from human endometrial side population cell lines.
Cervelló, I; Mas, A; Gil-Sanchis, C; Peris, L; Faus, A; Saunders, PT; Critchley, HO; Simón, C
PloS one
6
e21221
2011
Show Abstract
Endometrial regeneration is mediated, at least in part, by the existence of a specialized somatic stem cell (SSC) population recently identified by several groups using the side population (SP) technique. We previously demonstrated that endometrial SP displays genotypic, phenotypic and the functional capability to develop human endometrium after subcutaneous injection in NOD-SCID mice. We have now established seven human endometrial SP (hESP) cell lines (ICE 1-7): four from the epithelial and three from the stromal fraction, respectively. SP cell lines were generated under hypoxic conditions based on their cloning efficiency ability, cultured for 12-15 passages (20 weeks) and cryopreserved. Cell lines displayed normal 46XX karyotype, intermediate telomerase activity pattern and expressed mRNAs encoding proteins that are considered characteristic of undifferentiated cells (Oct-4, GDF3, DNMT3B, Nanog, GABR3) and those of mesodermal origin (WT1, Cardiac Actin, Enolase, Globin, REN). Phenotype analysis corroborated their epithelial (CD9+) or stromal (vimentin+) cell origin and mesenchymal (CD90+, CD73+ and CD45⁻) attributes. Markers considered characteristic of ectoderm or endoderm were not detected. Cells did not express either estrogen receptor alpha (ERα) or progesterone receptor (PR). The hESP cell lines were able to differentiate in vitro into adipocytes and osteocytes, which confirmed their mesenchymal origin. Finally, we demonstrated their ability to generate human endometrium when transplanted beneath the renal capsule of NOD-SCID mice. These findings confirm that SP cells exhibit key features of human endometrial SSC and open up new possibilities for the understanding of gynecological disorders such as endometriosis or Asherman syndrome. Our cell lines can be a valuable model to investigate new targets for endometrium proliferation in endometriosis. Full Text Article | 21712999
 |
Effect of cold-setting calcium- and magnesium phosphate matrices on protein expression in osteoblastic cells.
Andrea Ewald,Kerstin Helmschrott,Georg Knebl,Nazia Mehrban,Liam M Grover,Uwe Gbureck
Journal of biomedical materials research. Part B, Applied biomaterials
96
2011
Show Abstract
Bone loss due to accidents or tissue diseases requires replacement of the structure by either autografts, allografts, or artificial materials. Reactive cements, which are based on calcium phosphate chemistry, are commonly used in nonload bearing areas such as the craniofacial region. Some of these materials are resorbed by the host under physiological conditions and replaced by bone. The aim of this study was to test different calcium and magnesium cement composites in vitro for their use as bone substitution material. Phase composition of calcium deficient hydroxyapatite (Ca(9) (PO(4) )(5) HPO(4) OH), brushite (CaHPO(4) ·2H(2) O), and struvite (MgNH(4) PO(4) ·6H(2) O) specimens has been determined by means of X-ray diffraction, and compressive strength was measured. Cell growth and activity of osteoblastic cells (MG 63) on the different surfaces was determined, and the expression of bone marker proteins was analyzed by western blotting. Cell activity normalized to cell number revealed higher activity of the osteoblasts on brushite and struvite when compared to hydroxyapatite and also the expression of osteoblastic marker proteins was highest on brushite scaffolds. While brushite sets under acidic conditions, formation of struvite occurs under physiological pH, similar to hydroxyapatite cements, providing the possibility of additional modifications with proteins or other active components. | 21210513
 |
Differentiation of mesenchymal stem cells onto highly adherent radio frequency-sputtered carbonated hydroxylapatite thin films.
Sima, Livia E, et al.
J Biomed Mater Res A, 95: 1203-14 (2010)
2010
Show Abstract
In this work, an improved version of the radio frequency magnetron sputtering (RF-MS) technique was used to prepare highly adherent B-type carbonated hydroxylapatite (B-CHA) thin films. Fourier transform infrared spectroscopy (FTIR) and grazing incidence X-ray diffraction studies proved that the coatings maintained the composition and revealed the polycrystalline structure of HA. Scanning electron microscopy analysis showed that the CHA films are rough and exhibit a homogeneous microstructure. Energy-dispersive X-ray spectroscopy (EDX) mapping demonstrated a uniform distribution of the Ca and P cations while a Ca/P ratio of 1.8 was found. In addition, the FTIR experiments showed a remarkable reproducibility of the nanostructures. Human mesenchymal stem cells (hMSCs), in vitro differentiated osteoblasts, and explanted bone cells were grown over the surface of CHA coatings for periods between a few hours and 21 days. Osteoprogenitor cells maintained viability and characteristic morphology after adhesion on CHA coatings. Ki67-positive osteoblasts were the evidence of cell proliferation events. Cells showed positive staining for markers of osteoblast phenotype such as collagen type I, bone sialoprotein and osteonectin. Our data showed the formation of mineralized foci by differentiation of hMSCs to human primary osteoblasts after cultivation in osteogenic media on RF-sputtered films. The results demonstrate the capacity of B-type CHA coating to support MSCs adhesion and osteogenic differentiation ability. | 20939052
 |
Human endometrial side population cells exhibit genotypic, phenotypic and functional features of somatic stem cells.
Cervelló, I; Gil-Sanchis, C; Mas, A; Delgado-Rosas, F; Martínez-Conejero, JA; Galán, A; Martínez-Romero, A; Martínez, S; Navarro, I; Ferro, J; Horcajadas, JA; Esteban, FJ; O'Connor, JE; Pellicer, A; Simón, C
PloS one
5
e10964
2010
Show Abstract
During reproductive life, the human endometrium undergoes around 480 cycles of growth, breakdown and regeneration should pregnancy not be achieved. This outstanding regenerative capacity is the basis for women's cycling and its dysfunction may be involved in the etiology of pathological disorders. Therefore, the human endometrial tissue must rely on a remarkable endometrial somatic stem cells (SSC) population. Here we explore the hypothesis that human endometrial side population (SP) cells correspond to somatic stem cells. We isolated, identified and characterized the SP corresponding to the stromal and epithelial compartments using endometrial SP genes signature, immunophenotyping and characteristic telomerase pattern. We analyzed the clonogenic activity of SP cells under hypoxic conditions and the differentiation capacity in vitro to adipogenic and osteogenic lineages. Finally, we demonstrated the functional capability of endometrial SP to develop human endometrium after subcutaneous injection in NOD-SCID mice. Briefly, SP cells of human endometrium from epithelial and stromal compartments display genotypic, phenotypic and functional features of SSC. Full Text Article | 20585575
 |
Osteoblast response to biomimetically altered titanium surfaces.
J Barbara Nebe, Lenka Müller, Frank Lüthen, Andrea Ewald, Claudia Bergemann, Egle Conforto, Frank A Müller, J Barbara Nebe, Lenka Müller, Frank Lüthen, Andrea Ewald, Claudia Bergemann, Egle Conforto, Frank A Müller
Acta biomaterialia
4
1985-95
2008
Show Abstract
Bioinert titanium (Ti) materials are generally encapsulated by fibrous tissue after implantation into the living body. To improve the bone-bonding ability of Ti implants, we activated commercially pure titanium (cpTi) by a simple chemical pre-treatment in HCl and NaOH. Subsequently, we exposed the treated samples to simulated body fluid (SBF) for 2 (TiCT) and 14 days (TiHCA), respectively, to mimic the early stages of bone bonding and to investigate the in vitro response of osteoblasts on thus altered biomimetic surfaces. Sample surfaces were characterized by scanning electron microscopy, energy-dispersive X-ray analysis, cross-sectional transmission electron microscopy analyses, Fourier transform infrared and Raman spectroscopy. It was shown that the efflorescence consisting of sodium titanate that is present on pre-treated cpTi surfaces transformed to calcium titanate after 2 days in SBF. After 14 days in SBF a homogeneous biomimetic apatite layer precipitated. Human osteoblasts (MG-63) revealed a well spread morphology on both functionalized Ti surfaces. On TiCT, the gene expression of the differentiation proteins alkaline phosphatase (ALP) and bone sialo protein was increased after 2 days. On both TiCT and TiHCA, the collagen I and ALP expression on the protein level was enhanced at 7 and 14 days. The TiCT and the TiHCA surfaces reveal the tendency to increase the differentiated cell function of MG-63 osteoblasts. Thus, chemical pre-treatment of titanium seems to be a promising method to generate osteoconductive surfaces. | 18595788
 |