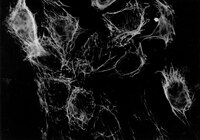
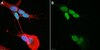
Transcriptional profiling of ectoderm specification to keratinocyte fate in human embryonic stem cells.
Tadeu, AM; Lin, S; Hou, L; Chung, L; Zhong, M; Zhao, H; Horsley, V
PloS one
10
e0122493
2015
Show Abstract
In recent years, several studies have shed light into the processes that regulate epidermal specification and homeostasis. We previously showed that a broad-spectrum γ-secretase inhibitor DAPT promoted early keratinocyte specification in human embryonic stem cells triggered to undergo ectoderm specification. Here, we show that DAPT accelerates human embryonic stem cell differentiation and induces expression of the ectoderm protein AP2. Furthermore, we utilize RNA sequencing to identify several candidate regulators of ectoderm specification including those involved in epithelial and epidermal development in human embryonic stem cells. Genes associated with transcriptional regulation and growth factor activity are significantly enriched upon DAPT treatment during specification of human embryonic stem cells to the ectoderm lineage. The human ectoderm cell signature identified in this study contains several genes expressed in ectodermal and epithelial tissues. Importantly, these genes are also associated with skin disorders and ectodermal defects, providing a platform for understanding the biology of human epidermal keratinocyte development under diseased and homeostatic conditions. | 25849374
 |
Generation of iPS Cells from Human Hair Follice Dermal Papilla Cells.
Muchkaeva, IA; Dashinimaev, EB; Artyuhov, AS; Myagkova, EP; Vorotelyak, EA; Yegorov, YY; Vishnyakova, KS; Kravchenko, IE; Chumakov, PM; Terskikh, VV; Vasiliev, AV
Acta naturae
6
45-53
2014
Show Abstract
Dermal papilla (DP) cells are unique regional stem cells of the skin that induce formation of a hair follicle and its regeneration cycle. DP are multipotent stem cells; therefore we supposed that the efficiency of DPC reprogramming could exceed that of dermal fibroblasts reprogramming. We generated induced pluripotent stem cells from human DP cells using lentiviral transfection with Oct4, Sox2, Klf4, and c-Myc, and cultivation of cells both in a medium supplemented with valproic acid and at a physiological level of oxygen (5%). The efficiency of DP cells reprogramming was ~0.03%, while the efficiency of dermal fibroblast reprogramming under the same conditions was ~0.01%. Therefore, we demonstrated the suitability of DP cells as an alternative source of iPS cells. | 24772326
 |
Notch signaling represses p63 expression in the developing surface ectoderm.
Tadeu, AM; Horsley, V
Development (Cambridge, England)
140
3777-86
2013
Show Abstract
The development of the mature epidermis requires a coordinated sequence of signaling events and transcriptional changes to specify surface ectodermal progenitor cells to the keratinocyte lineage. The initial events that specify epidermal keratinocytes from ectodermal progenitor cells are not well understood. Here, we use both developing mouse embryos and human embryonic stem cells (hESCs) to explore the mechanisms that direct keratinocyte fate from ectodermal progenitor cells. We show that both hESCs and murine embryos express p63 before keratin 14. Furthermore, we find that Notch signaling is activated before p63 expression in ectodermal progenitor cells. Inhibition of Notch signaling pharmacologically or genetically reveals a negative regulatory role for Notch signaling in p63 expression during ectodermal specification in hESCs or mouse embryos, respectively. Taken together, these data reveal a role for Notch signaling in the molecular control of ectodermal progenitor cell specification to the epidermal keratinocyte lineage. | 23924630
 |
Effects of ligustrazine on ureteral obstruction-induced renal tubulointerstitial fibrosis.
Xiao-Peng Yuan,Long-Shan Liu,Qian Fu,Chang-Xi Wang
Phytotherapy research : PTR
26
2012
Show Abstract
Ligustrazine (LIG) is a purified and chemically identified component of the Chinese herb Ligusticum wallichii Franchat. It is a potent blocker of vasoconstriction and has strong scavenger of oxygen free radicals. We investigated the effect of LIG on renal tubulointerstitial fibrosis using a rat model of unilateral ureteral obstruction. Ligustrazine treatment significantly reduced the scores of interstitial collagen deposition, amounts of hydroxyproline, the density of myofibroblasts and macrophages, and amounts of transforming growth factor-β1 (TGF-β1) and connective tissue growth factor (CTGF) compared with their level in a saline-treated control group. Using quantitative polymerase chain reaction we found that LIG treatment significantly reduced the mRNA expression of TGF-β1, CTGF, monocyte chemoattractant protein-1 and osteopontin. Moreover, the mRNA expression of hepatocyte growth factor and bone morphogenetic protein-7 were significantly increased by LIG. In vitro, LIG inhibited the TGF-β1-induced loss of cytokeratin-18 expression and de novo increase of the expression of α-smooth muscle actin of HK-2 cells in a dose-dependent manner, which suggested that LIG could restrain the process of epithelial-myofibroblast transition of tubular epithelial cells. This study indicates that LIG can attenuate renal tubulointerstitial fibrosis. It might be useful as a potential candidate in the treatment of chronic renal diseases. | 22006851
 |
Modulation of choroidal neovascularization by subretinal injection of retinal pigment epithelium and polystyrene microbeads.
Schmack, I; Berglin, L; Nie, X; Wen, J; Kang, SJ; Marcus, AI; Yang, H; Lynn, MJ; Kapp, JA; Grossniklaus, HE
Molecular vision
15
146-61
2009
Show Abstract
The study was conducted to create a rapidly developing and reproducible animal model of subretinal choroidal neovascularization (CNV) that allows a time-dependent evaluation of growth dynamics, histopathologic features, and cytokine expression.C57BL/6 and chemoattractant leukocyte protein-2 deficient (DeltaCcl-2) mice were studied. Mice received single or combined subretinal injections of cultured retinal pigment epithelium (RPE; C57BL/6-derived), polystyrene microbeads, or phosphate buffer solution (PBS). Fluorescence angiograms were performed over a period of 3 weeks. Mice were euthanized on post inoculation day 3, 7, 10, 14, or 21, and their eyes were evaluated by light, confocal, and electron microscopy.CNV membranes occurred in all study groups with an overall incidence of 94.3%. They extended in the subretinal space through central breaks in Bruch's membrane. CNV lesions were characterized by dynamic changes such as initiation, active inflammatory, and involution stages. CNV thickness peaked around PI day 7 and was greater in mice that received combined injections of RPE and microbeads or RPE cells alone. Small lesions developed in the control groups (microbeads or PBS only), in DeltaCcl-2, and old C57BL/6 mice. Variable expression of cytokines and growth factors was detected within the membranes.Our murine model represents a reliable approach inducing CNV growth by subretinal injection of either RPE cells alone or RPE cells and microbeads. The development of CNV lesions is a dynamic process that relies in part on macrophage trafficking and age. Full Text Article | 19158960
 |
Establishment of conditionally immortalized epithelial cell lines from the intestinal tissue of adult normal and transgenic mice.
Whitehead, RH; Robinson, PS
American journal of physiology. Gastrointestinal and liver physiology
296
G455-60
2009
Show Abstract
It has proved to be impossible to culture epithelial cells from the gastrointestinal tract of adult animals. Researchers have had to use either cell lines derived from newborn rat small intestine or colon carcinoma cell lines that have retained some of the properties of the gastrointestinal mucosa. We have described a method for establishing conditionally immortalized cell lines from the stomach, small intestine, colon, pancreas, and liver from tissue obtained from a transgenic mouse strain carrying a temperature-sensitive mutant of the SV40 large T gene (the "Immortomouse"). This immortalizing gene has proved to be useful for establishing cell lines from a number of transgenic mice following crossbreeding of the Immortomouse with the transgenic mouse of interest. These cell lines are being used in numerous studies. In this review we describe the methods for developing such lines and list the range of cell lines that have been developed from colon, small intestine, stomach, liver, and pancreas of a number of transgenic mice. Full Text Article | 19109407
 |
[Extraembryonic endoderm stem cell lines from common voles of the genus Microtus].
A I Shevchenko,V V Demina,N A Mazurok,A I Zhelezova,Ia R Efremov,a G Shilov,A I Shevela,A V Belevantseva,V V Vlasov,S M Zakiian
Genetika
44
2008
Show Abstract
Twenty-eight independent extraembryonic endoderm (XEN) stem cell lines have been obtained from morula and blastocyst cells of common voles. Most cell lines form very few cell-cell contacts when growing and morphologically correspond to the XEN that were earlier described in mice. In addition, XEN cell lines with atypical morphology forming colonies have been obtained for the first time. Both types of XEN lines rapidly proliferate, retain their morphology and karyotype during more than 25 passages in cell culture, and express genes characteristic of XEN. One of two X chromosomes in XEN lines with karyotype XX has been shown to be inactive and associated with the Xist gene transcript. It has been demonstrated that the paternal X chromosome is inactive. | 19137730
 |
DeltaNp63 is essential for epidermal commitment of embryonic stem cells.
Alain Medawar,Thierry Virolle,Philippe Rostagno,Stéphanie de la Forest-Divonne,Karen Gambaro,Matthieu Rouleau,Daniel Aberdam
PloS one
3
2008
Show Abstract
In vivo studies have demonstrated that p63 plays complex and pivotal roles in pluristratified squamous epithelial development, but its precise function and the nature of the isoform involved remain controversial. Here, we investigate the role of p63 in epithelial differentiation, using an in vitro ES cell model that mimics the early embryonic steps of epidermal development. We show that the DeltaNp63 isoform is activated soon after treatment with BMP-4, a morphogen required to commit differentiating ES cells from a neuroectodermal to an ectodermal cell fate. DeltaNp63 gene expression remains high during epithelial development. P63 loss of function drastically prevents ectodermal cells to commit to the K5/K14-positive stratified epithelial pathway while gain of function experiments show that DeltaNp63 allows this commitment. Interestingly, other epithelial cell fates are not affected, allowing the production of K5/K18-positive epithelial cells. Therefore, our results demonstrate that DeltaNp63 may be dispensable for some epithelial differentiation, but is necessary for the commitment of ES cells into K5/K14-positive squamous stratified epithelial cells. Full Text Article | 18927616
 |
BMP-4 induces a Smad-dependent apoptotic cell death of mouse embryonic stem cell-derived neural precursors.
Gambaro, K; Aberdam, E; Virolle, T; Aberdam, D; Rouleau, M
Cell death and differentiation
13
1075-87
2006
Show Abstract
Embryonic ectoderm is fated to become either neural or epidermal, depending on patterning processes that occur before and during gastrulation. It has been stated that epidermal commitment proceeds from a bone morphogenetic protein-4 (BMP-4)-dependent inhibition of dorsal ectoderm neuralization. We recently demonstrated that murine embryonic stem (ES) cells treated with BMP-4 undergo effective keratinocyte commitment and epidermogenesis. Focusing on the precise role of BMP-4 in the early choice between neural and epidermal commitment, we show here that BMP-4 treatment of ES cells leads to a dramatic apoptotic death of Sox-1+ neural precursors with concomitant epidermal engagement. In addition, neutralization of the Smad pathway prevents both the BMP-4 apoptotic process and the inhibition of neural differentiation. Our results suggest that, in mammals, BMP-4, as an active inducer of epidermal commitment, interferes with the survival of neural precursors through induction of their apoptotic cell death. | 16311513
 |
Identification of pulmonary Oct-4+ stem/progenitor cells and demonstration of their susceptibility to SARS coronavirus (SARS-CoV) infection in vitro.
Ling, TY; Kuo, MD; Li, CL; Yu, AL; Huang, YH; Wu, TJ; Lin, YC; Chen, SH; Yu, J
Proceedings of the National Academy of Sciences of the United States of America
103
9530-5
2006
Show Abstract
In this study, we report a serum-free culture system for primary neonatal pulmonary cells that can support the growth of octamer-binding transcription factor 4+ (Oct-4+) epithelial colonies with a surrounding mesenchymal stroma. In addition to Oct-4, these cells also express other stem cell markers such as stage-specific embryonic antigen 1 (SSEA-1), stem cell antigen 1 (Sca-1), and Clara cell secretion protein (CCSP) but not c-Kit, CD34, and p63, indicating that they represent a subpopulation of Clara cells that have been implicated as lung stem/progenitor cells in lung injury models. These colony cells can be kept for weeks in primary cultures and undergo terminal differentiation to alveolar type-2- and type-1-like pneumocytes sequentially when removed from the stroma. In addition, we have demonstrated the presence of Oct-4+ long-term BrdU label-retaining cells at the bronchoalveolar junction of neonatal lung, providing a link between the Oct-4+ cells in vivo and in vitro and strengthening their identity as putative neonatal lung stem/progenitor cells. Lastly, these Oct-4+ epithelial colony cells, which also express angiotensin-converting enzyme 2, are the target cells for severe acute respiratory syndrome coronavirus infection in primary cultures and support active virus replication leading to their own destruction. These observations imply the possible involvement of lung stem/progenitor cells, in addition to pneumocytes, in severe acute respiratory syndrome coronavirus infection, accounting for the continued deterioration of lung tissues and apparent loss of capacity for lung repair. | 16772384
 |