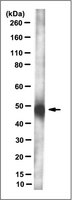
MLN64 is involved in actin-mediated dynamics of late endocytic organelles.
Hölttä-Vuori, M; Alpy, F; Tanhuanpää, K; Jokitalo, E; Mutka, AL; Ikonen, E
Molecular biology of the cell
16
3873-86
2005
Show Abstract
MLN64 is a late endosomal cholesterol-binding membrane protein of an unknown function. Here, we show that MLN64 depletion results in the dispersion of late endocytic organelles to the cell periphery similarly as upon pharmacological actin disruption. The dispersed organelles in MLN64 knockdown cells exhibited decreased association with actin and the Arp2/3 complex subunit p34-Arc. MLN64 depletion was accompanied by impaired fusion of late endocytic organelles and delayed cargo degradation. MLN64 overexpression increased the number of actin and p34-Arc-positive patches on late endosomes, enhanced the fusion of late endocytic organelles in an actin-dependent manner, and stimulated the deposition of sterol in late endosomes harboring the protein. Overexpression of wild-type MLN64 was capable of rescuing the endosome dispersion in MLN64-depleted cells, whereas mutants of MLN64 defective in cholesterol binding were not, suggesting a functional connection between MLN64-mediated sterol transfer and actin-dependent late endosome dynamics. We propose that local sterol enrichment by MLN64 in the late endosomal membranes facilitates their association with actin, thereby governing actin-dependent fusion and degradative activity of late endocytic organelles. | 15930133
 |
The steroidogenic acute regulatory protein homolog MLN64, a late endosomal cholesterol-binding protein.
Alpy, F; Stoeckel, ME; Dierich, A; Escola, JM; Wendling, C; Chenard, MP; Vanier, MT; Gruenberg, J; Tomasetto, C; Rio, MC
The Journal of biological chemistry
276
4261-9
2001
Show Abstract
MLN64 is a transmembrane protein that shares homology with the cholesterol binding domain (START domain) of the steroidogenic acute regulatory protein. The steroidogenic acute regulatory protein is located in the inner membrane of mitochondria, where it facilitates cholesterol import into the mitochondria. Crystallographic analysis showed that the START domain of MLN64 is a cholesterol-binding domain. The present work was undertaken to determine which step of the intracellular cholesterol pathway MLN64 participates in. Using immunocytofluorescence, MLN64 colocalizes with LBPA, a lipid found specifically in late endosomes. Electron microscopy indicates that MLN64 is restricted to the limiting membrane of late endosomes. Microinjection or endocytosis of specific antibodies shows that the START domain of MLN64 is cytoplasmic. Deletion and mutagenesis experiments demonstrate that the amino-terminal part of MLN64 is responsible for its addressing. Although this domain does not contain conventional dileucine- or tyrosine-based targeting signals, we show that a dileucine motif (Leu(66)-Leu(67)) and a tyrosine residue (Tyr(89)) are critical for the targeting or the proper folding of the molecule. Finally, MLN64 colocalizes with cholesterol and Niemann Pick C1 protein in late endosomes. However, complementation assays show that MLN64 is not involved in the Niemann Pick C2 disease which, results in cholesterol lysosomal accumulation. Together, our results show that MLN64 plays a role at the surface of the late endosomes, where it might shuttle cholesterol from the limiting membrane to cytoplasmic acceptor(s). | 11053434
 |
MLN64 exhibits homology with the steroidogenic acute regulatory protein (STAR) and is over-expressed in human breast carcinomas.
Moog-Lutz, C; Tomasetto, C; Régnier, CH; Wendling, C; Lutz, Y; Muller, D; Chenard, MP; Basset, P; Rio, MC
International journal of cancer. Journal international du cancer
71
183-91
1997
Show Abstract
The MLN64 gene, which is localized in q12-q21 of the human chromosome 17, encodes a novel protein containing 2 distinct domains. At the N-terminal, MLN64 exhibits a potential trans-membrane region, while at the C-terminal, it shares homology with the F26F4.4 protein of Coenorhabditis elegans and the steroidogenic acute regulatory (StAR) protein, a mitochondrial protein which is involved in steroid-hormone synthesis. By comparing the C-terminal part of these proteins, we defined a novel protein domain, which we termed SHD for "StAR Homology Domain". Of the 93 primary invasive breast carcinomas that were examined, 14 were found to over-express MLN64. These 14 tumors also expressed high c-erbB-2 transcript levels, which were not detected in the MLN64-negative tumors. MLN64 mRNA and protein were specifically detected in malignant cells of breast carcinomas. MLN64 protein was localized within bundle-like structures distributed throughout the cell cytoplasm and condensed in a perinuclear patch, suggesting an association with a specific cell compartment. When the N-terminal part of MLN64 was deleted, MLN64 was uniformly distributed in the cell cytoplasm, indicating that N-terminal part is involved in the specific cytoplasmic localization of MLN64. The homology between the C-terminal part of MLN64 and the functional StAR domain (SHD) suggests that MLN64 and StAR, although distributed in different cellular compartments, may both play a role in steroidogenesis. In this case, the high levels of MLN64 observed in some breast carcinomas could contribute to the progression of these tumors through increased intratumoral steroidogenesis. | 9139840
 |
MLN64 contains a domain with homology to the steroidogenic acute regulatory protein (StAR) that stimulates steroidogenesis.
Watari, H; Arakane, F; Moog-Lutz, C; Kallen, CB; Tomasetto, C; Gerton, GL; Rio, MC; Baker, ME; Strauss, JF
Proceedings of the National Academy of Sciences of the United States of America
94
8462-7
1997
Show Abstract
MLN64 is a protein that is highly expressed in certain breast carcinomas. The C terminus of MLN64 shares significant homology with the steroidogenic acute regulatory protein (StAR), which plays a key role in steroid hormone biosynthesis by enhancing the intramitochondrial translocation of cholesterol to the cholesterol side-chain cleavage enzyme. We tested the ability of MLN64 to stimulate steroidogenesis by using COS-1 cells cotransfected with plasmids expressing the human cholesterol side-chain cleavage enzyme system and wild-type and mutant MLN64 proteins. Wild-type MLN64 increased pregnenolone secretion in this system 2-fold. The steroidogenic activity of MLN64 was found to reside in the C terminus of the protein, because constructs from which the C-terminal StAR homology domain was deleted had no steroidogenic activity. In contrast, removal of N-terminal sequences increased MLN64's steroidogenesis-enhancing activity. MLN64 mRNA was found in many human tissues, including the placenta and brain, which synthesize steroid hormones but do not express StAR. Western blot analysis revealed the presence of lower molecular weight immunoreactive MLN64 species that contain the C-terminal sequences in human tissues. Homologs of both MLN64 and StAR were identified in Caenorhabditis elegans, indicating that the two proteins are ancient. Mutations that inactivate StAR were correlated with amino acid residues that are identical or similar among StAR and MLN64, indicating that conserved motifs are important for steroidogenic activity. We conclude that MLN64 stimulates steroidogenesis by virtue of its homology to StAR. | 9237999
 |