IM64 Anti-MMP-13 (Ab-3) Mouse mAb (181-14G11)
Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| Host |
|---|
| M |
| Description | |
|---|---|
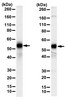
| Overview | Recognizes the ~54-60 kDa latent and the ~48 kDa active forms of MMP-13. |
| Catalogue Number | IM64 |
| Brand Family | Calbiochem® |
| Synonyms | Anti-Collagenase-3, Anti-Matrix Metalloproteinase 13 |
| Biological Information | |
|---|---|
| Immunogen | recombinant human pro-MMP-13 |
| Immunogen | Human |
| Clone | 181-14G11 |
| Host | Mouse |
| Isotype | IgG₁ |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Storage and Shipping Information | |
|---|---|
| Ship Code | Blue Ice Only |
| Toxicity | Standard Handling |
| Storage | -20°C |
| Do not freeze | Ok to freeze |
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| IM64 | 0 |
Documentation
References
| Reference overview |
|---|
| Shlopov, B., et al. 1997. Arthritis Rheum. 40, 2065. Knauper V., et al. 1996. J. Biol. Chem. 271, 1544. Freije, J.M.P., et al. 1994. J. Biol. Chem. 269, 16766. Cottam, D. W. and Rees, R. C., 1993. Intl. J. Oncol. 2, 861. Stetler-Stevenson, et al. 1993. FASEB J. 7, 1434. Woessner, J. F., 1991. FASEB J. 5, 2145. Liotta, L. A. and Stetler-Stevenson, W. G., 1990. In Seminars in Cancer Biology, ed. M. M. Gottesman. Vol. 1, 99. |