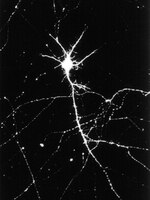
Na+/K+ ATPase α1 and α3 isoforms are differentially expressed in α- and γ-motoneurons.
Edwards, IJ; Bruce, G; Lawrenson, C; Howe, L; Clapcote, SJ; Deuchars, SA; Deuchars, J
The Journal of neuroscience : the official journal of the Society for Neuroscience
33
9913-9
2013
Show Abstract
The Na(+)/K(+) ATPase (NKA) is an essential membrane protein underlying the membrane potential in excitable cells. Transmembrane ion transport is performed by the catalytic α subunits (α1-4). The predominant subunits in neurons are α1 and α3, which have different affinities for Na(+) and K(+), impacting on transport kinetics. The exchange rate of Na(+)/K(+) markedly influences the activity of the neurons expressing them. We have investigated the distribution and function of the main isoforms of the α subunit expressed in the mouse spinal cord. NKAα1 immunoreactivity (IR) displayed restricted labeling, mainly confined to large ventral horn neurons and ependymal cells. NKAα3 IR was more widespread in the spinal cord, again being observed in large ventral horn neurons, but also in smaller interneurons throughout the dorsal and ventral horns. Within the ventral horn, the α1 and α3 isoforms were mutually exclusive, with the α3 isoform in smaller neurons displaying markers of γ-motoneurons and α1 in α-motoneurons. The α3 isoform was also observed within muscle spindle afferent neurons in dorsal root ganglia with a higher proportion at cervical versus lumbar regions. We confirmed the differential expression of α subunits in motoneurons electrophysiologically in neonatal slices of mouse spinal cord. γ-Motoneurons were excited by bath application of low concentrations of ouabain that selectively inhibit NKAα3 while α-motoneurons were insensitive to these low concentrations. The selective expression of NKAα3 in γ-motoneurons and muscle spindle afferents, which may affect excitability of these neurons, has implications in motor control and disease states associated with NKAα3 dysfunction. | Western Blotting | 23761886
 |
Na,K-ATPase alpha isoforms at the blood-cerebrospinal fluid-trigeminal nerve and blood-retina interfaces in the rat.
Arakaki, X; McCleary, P; Techy, M; Chiang, J; Kuo, L; Fonteh, AN; Armstrong, B; Levy, D; Harrington, MG
Fluids and barriers of the CNS
10
14
2013
Show Abstract
Cerebrospinal fluid (CSF) sodium concentration increases during migraine attacks, and both CSF and vitreous humor sodium increase in the rat migraine model. The Na,K-ATPase is a probable source of these sodium fluxes. Since Na,K-ATPase isoforms have different locations and physiological roles, our objective was to establish which alpha isoforms are present at sites where sodium homeostasis is disrupted.Specific Na,K-ATPase alpha isoforms were identified in rat tissues by immunohistochemistry at the blood-CSF barrier at the choroid plexus, at the blood-CSF-trigeminal barrier at the meninges, at the blood-retina barrier, and at the blood-aqueous barrier at the ciliary body. Calcitonin gene-related peptide (CGRP), occludin, or von Willibrand factor (vWF) were co-localized with Na,K-ATPase to identify trigeminal nociceptor fibers, tight junctions, and capillary endothelial cells respectively.The Na,K-ATPase alpha-2 isoform is located on capillaries and intensely at nociceptive trigeminal nerve fibers at the meningeal blood-CSF-trigeminal barrier. Alpha-1 and -3 are lightly expressed on the trigeminal nerve fibers but not at capillaries. Alpha-2 is expressed at the blood-retina barriers and, with alpha-1, at the ciliary body blood aqueous barrier. Intense apical membrane alpha-1 was associated with moderate cytoplasmic alpha-2 expression at the choroid plexus blood-CSF barrier.Na,K-ATPase alpha isoforms are present at the meningeal, choroid plexus, and retinal barriers. Alpha-2 predominates at the capillary endothelial cells in the meninges and retinal ganglion cell layer. | | 23497725
 |
Mutation I810N in the alpha3 isoform of Na+,K+-ATPase causes impairments in the sodium pump and hyperexcitability in the CNS.
Clapcote, SJ; Duffy, S; Xie, G; Kirshenbaum, G; Bechard, AR; Rodacker Schack, V; Petersen, J; Sinai, L; Saab, BJ; Lerch, JP; Minassian, BA; Ackerley, CA; Sled, JG; Cortez, MA; Henderson, JT; Vilsen, B; Roder, JC
Proceedings of the National Academy of Sciences of the United States of America
106
14085-90
2009
Show Abstract
In a mouse mutagenesis screen, we isolated a mutant, Myshkin (Myk), with autosomal dominant complex partial and secondarily generalized seizures, a greatly reduced threshold for hippocampal seizures in vitro, posttetanic hyperexcitability of the CA3-CA1 hippocampal pathway, and neuronal degeneration in the hippocampus. Positional cloning and functional analysis revealed that Myk/+ mice carry a mutation (I810N) which renders the normally expressed Na(+),K(+)-ATPase alpha3 isoform inactive. Total Na(+),K(+)-ATPase activity was reduced by 42% in Myk/+ brain. The epilepsy in Myk/+ mice and in vitro hyperexcitability could be prevented by delivery of additional copies of wild-type Na(+),K(+)-ATPase alpha3 by transgenesis, which also rescued Na(+),K(+)-ATPase activity. Our findings reveal the functional significance of the Na(+),K(+)-ATPase alpha3 isoform in the control of epileptiform activity and seizure behavior. | | 19666602
 |
Neurons and astroglia express distinct subsets of Na,K-ATPase alpha and beta subunits.
Cameron, R, et al.
Brain Res. Mol. Brain Res., 21: 333-43 (1994)
1994
Show Abstract
We have analyzed the expression pattern of Na,K-ATPase alpha and beta subunit isoforms within the rodent and primate central nervous system. Membrane fractions prepared from rat cerebral cortical type-1 astrocytes and rat cerebellar granule and hippocampal neurons were characterized by immunoblot analyses using a panel of alpha and beta subunit isoform-specific antisera. Each cell type was found to express the alpha 1 isoform but showed differences in the expression of other subunits. Cortical astrocytes displayed alpha 2 and beta 2 subunits, whereas cerebellar granule neurons showed expression of alpha 3 and beta 1 subunits. All three alpha subunit isotypes were detected in hippocampal neurons. A survey of the immunofluorescent staining pattern of the alpha 3 subunit in rat and monkey brain confirmed that expression of this Na,K-ATPase alpha subunit isoform was restricted exclusively to neurons. These results suggest that both neurons and astrocytes express multiple, yet distinct, Na,K-ATPase isoenzymes. The identification of cell types expressing limited combinations of alpha and beta subunits should provide a framework for understanding the physiological significance of Na,K-ATPase isoenzyme diversity and may provide useful tools for the analysis of cell lineage in the mammalian central nervous system. | | 8170354
 |
Differential expression and enzymatic properties of the Na+,K(+)-ATPase alpha 3 isoenzyme in rat pineal glands.
Shyjan, A W, et al.
Proc. Natl. Acad. Sci. U.S.A., 87: 1178-82 (1990)
1990
Show Abstract
We have used immunoblotting and biochemical techniques to analyze expression of Na+,K(+)-ATPase alpha and beta subunits in rat pineal glands. Western blot analysis of pineal microsomal membrane fractions with antisera specific for each of the three rat alpha and two rat beta subunits revealed similar levels of expression of alpha 1 and alpha 3 subunits in pineal glands of 5-day-old rats. High levels of alpha 3 and beta 2 subunits and low levels of alpha 1 subunits were detected in adult glands. No alpha 2 or beta 1 subunits were detectable at either developmental stage. Examination of the enzymatic properties of the pineal gland alpha 3 isoform suggests that this enzyme is a ouabain-sensitive ATPase whose activity is dependent upon Na+ and K+. This ATPase exhibited a lower apparent Km for Na+ than the kidney alpha 1 isoenzyme and did not show positive cooperative Na+ activation. Our results suggest that the activity of the Na+,K(+)-ATPase alpha 3 isoenzyme may be adapted to function under conditions of hyperpolarizing transmembrane potentials. | | 2153972
 |