Tumor-specific chromosome mis-segregation controls cancer plasticity by maintaining tumor heterogeneity.
Hu, Y; Ru, N; Xiao, H; Chaturbedi, A; Hoa, NT; Tian, XJ; Zhang, H; Ke, C; Yan, F; Nelson, J; Li, Z; Gramer, R; Yu, L; Siegel, E; Zhang, X; Jia, Z; Jadus, MR; Limoli, CL; Linskey, ME; Xing, J; Zhou, YH
PloS one
8
e80898
2013
Show Abstract
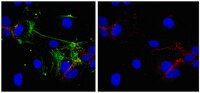
Aneuploidy with chromosome instability is a cancer hallmark. We studied chromosome 7 (Chr7) copy number variation (CNV) in gliomas and in primary cultures derived from them. We found tumor heterogeneity with cells having Chr7-CNV commonly occurs in gliomas, with a higher percentage of cells in high-grade gliomas carrying more than 2 copies of Chr7, as compared to low-grade gliomas. Interestingly, all Chr7-aneuploid cell types in the parental culture of established glioma cell lines reappeared in single-cell-derived subcultures. We then characterized the biology of three syngeneic glioma cultures dominated by different Chr7-aneuploid cell types. We found phenotypic divergence for cells following Chr7 mis-segregation, which benefited overall tumor growth in vitro and in vivo. Mathematical modeling suggested the involvement of chromosome instability and interactions among cell subpopulations in restoring the optimal equilibrium of tumor cell types. Both our experimental data and mathematical modeling demonstrated that the complexity of tumor heterogeneity could be enhanced by the existence of chromosomes with structural abnormality, in addition to their mis-segregations. Overall, our findings show, for the first time, the involvement of chromosome instability in maintaining tumor heterogeneity, which underlies the enhanced growth, persistence and treatment resistance of cancers. | Immunofluorescence | 24282558
 |
Elutriated stem cells derived from the adult bone marrow differentiate into insulin-producing cells in vivo and reverse chemical diabetes.
Svetlana Iskovich,Nitza Goldenberg-Cohen,Jerry Stein,Isaac Yaniv,Ina Fabian,Nadir Askenasy
Stem cells and development
21
2012
Show Abstract
An ongoing debate surrounds the existence of stem cells in the adult endowed with capacity to differentiate into multiple lineages. We examined the possibility that adult bone marrow cells participate in recovery from chemical diabetes through neogenesis of insulin-producing cells. Small-sized cells negative for lineage markers derived by counterflow centrifugal elutriation from the bone marrow were transplanted into mice made diabetic with streptozotocin and sublethal irradiation. These cells homed efficiently to the injured islets and contributed to tissue revascularization. Islet-homed CD45-negative donor cells identified by sex chromosomes downregulated GFP, expressed PDX-1 and proinsulin, and converted the hormone precursor to insulin. An estimated 7.6% contribution of newly formed insulin-producing cells to islet cellularity increased serum insulin and stabilized glycemic control starting at 5 weeks post-transplant and persisting for 20 weeks. Newly differentiated cells displayed normal diploid genotype and there was no evidence of fusion between the grafted stem cells or their myeloid progeny and injured β-cells. Considering the extensive functional incorporation of insulin-producing donor cells in the injured islets, we conclude that the adult bone marrow contains a subset of small cells endowed with plastic developmental capacity. | | 21457125
 |
Targeting Vascular Amyloid in Arterioles of Alzheimer Disease Transgenic Mice With Amyloid β Protein Antibody-Coated Nanoparticles.
Poduslo JF, Hultman KL, Curran GL, Preboske GM, Chamberlain R, Marjańska M, Garwood M, Jack CR Jr, Wengenack TM.
Journal of neuropathology and experimental neurology
70
653-61
2011
Show Abstract
The relevance of cerebral amyloid angiopathy (CAA) to the pathogenesis of Alzheimer disease (AD) and dementia in general emphasizes the importance of developing novel targeting approaches for detecting and treating cerebrovascular amyloid (CVA) deposits. We developed a nanoparticle-based technology that uses a monoclonal antibody against fibrillar human amyloid-β42 that is surface coated onto a functionalized phospholipid monolayer. We demonstrate that this conjugated nanoparticle binds to CVA deposits in arterioles of AD transgenic mice (Tg2576) after infusion into the external carotid artery using 3 different approaches. The first 2 approaches use a blood vessel enrichment of homogenized brain and a leptomeningeal vessel preparation from thin tangential brain slices from the surface of the cerebral cortex. Targeting of CVA by the antibody-coated nanoparticle was visualized using fluorescent lissamine rhodamine-labeled phospholipids in the nanoparticles, which were compared with fluorescent staining of the endothelial cells and amyloiddeposits using confocal laser scanning microscopy. The third approach used high-field strength magnetic resonance imaging of antibody-coated iron oxide nanoparticles after infusion into the externalcarotid artery. Dark foci of contrast enhancement in cortical arterioles were observed in T2*-weighted images of ex vivo AD mouse brains that correlated histologically with CVA deposits. The targeting ability of these nanoparticles to CVA provides opportunities for the prevention and treatment of CAA. | | 21760540
 |
Nestin-driven green fluorescent protein as an imaging marker for nascent blood vessels in mouse models of cancer.
Hoffman RM
Methods in molecular biology (Clifton, N.J.)
689
183-204
2011
Show Abstract
A transgenic mouse, in which the regulatory elements of the stem cell marker, nestin drive green fluorescent protein (ND-GFP), expresses GFP in nascent blood vessels. Red fluorescent protein (RFP)-expressing tumors transplanted to nestin-GFP mice enable specific visualization of nascent vessels in the growing tumors. The ND-GFP mouse was also utilized to develop a rapid in vivo/ex vivo fluorescent angiogenesis assay by implanting Gelfoam(®), a surgical sponge derived from pigskin, which was rapidly vascularized by fluorescent nascent blood vessels. Angiogenesis could be imaged and quantified when stimulated or inhibited by specific compounds in both tumors and Gelfoam(®). These fluorescent models can be used to study the early events of angiogenesis and to quantitatively determine efficacy of antiangiogenesis compounds. | | 21153793
 |
A color-coded orthotopic nude-mouse treatment model of brain-metastatic paralyzing spinal cord cancer that induces angiogenesis and neurogenesis.
K Hayashi, K Yamauchi, N Yamamoto, H Tsuchiya, K Tomita, M Bouvet, J Wessels, R M Hoffman, K Hayashi, K Yamauchi, N Yamamoto, H Tsuchiya, K Tomita, M Bouvet, J Wessels, R M Hoffman
Cell proliferation
42
75-82
2009
Show Abstract
OBJECTIVE: Cancer of the spinal cord is highly malignant and often leads to paralysis and death. A realistic mouse model would be an important benefit for the better understanding and treatment of spinal cord glioma. MATERIALS AND METHODS: To develop an imageable, patient-like model of this disease, U87 human glioma tumour fragments (expressing red fluorescent protein), were transplanted by surgical orthotopic implantation into the spinal cord of nontransgenic nude mice or transgenic nude mice expressing nestin-driven green fluorescent protein (ND-GFP). In ND-GFP mice, GFP is expressed in nascent blood vessels and neural stem cells. The animals were treated with temozolomide or vehicle control. RESULTS: The intramedullary spinal cord tumour grew at the primary site, caused hind-limb paralysis and also metastasized to the brain. Temozolomide inhibited tumour growth (P0.01) and prevented metastasis, as well as prevented paralysis in four mice and delayed paralysis in two mice of the six tested (P=0.005). In the ND-GFP-expressing host, ND-GFP cells staining positively for neuronal class III-beta-tubulin or CD31, surrounded the tumour. These results suggest that the tumour stimulated both neurogenesis and angiogenesis, respectively. CONCLUSION: A patient-like model of spinal cord glioma was thus developed, which can be used for the discovery of new agents, including those that inhibit invasion and metastasis of the disease as well as those that prevent paralysis. | | 19143765
 |
Dual-color imaging of tumor angiogenesis.
Robert M Hoffman, Robert M Hoffman, Robert M Hoffman
Methods in molecular biology (Clifton, N.J.)
515
1-17
2009
Show Abstract
Angiogenesis is a critical step in the process of tumor metastasis. Many models have been used to study this process, but they have been artificial and do not reflect the actual process that takes place in the human being. Our laboratory has developed realistic models of angiogenesis based on orthotopic transplantation of human tumors in mice. In order to make angiogenesis visible in real time, our laboratory has developed mouse models in which the blood vessels are labeled with green fluorescent protein (GFP) such that they can be visualized by vascularizing tumors expressing red fluorescent protein (RFP). A particularly valuable model is a nude mouse in which the promoter from the stem-cell-marker protein, nestin, drives the expression of GFP. In such transgenic mice, the nascent blood vessels, in contrast to the mature blood vessels, express GFP. This model, in which human tumors expressing RFP are implanted, has been used to test drugs for their antitumor and antiangiogenetic activity. We have observed for the first time the high antiangiogenetic efficacy of cancer drugs such as gemcitabine and doxorubicin. These models should prove very valuable in the discovery of new antiangiogenesis drugs. | | 19378118
 |
A novel eGFP-expressing immunodeficient mouse model to study tumor-host interactions.
Niclou, SP; Danzeisen, C; Eikesdal, HP; Wiig, H; Brons, NH; Poli, AM; Svendsen, A; Torsvik, A; Enger, PØ; Terzis, JA; Bjerkvig, R
FASEB journal : official publication of the Federation of American Societies for Experimental Biology
22
3120-8
2008
Show Abstract
A NOD/Scid mouse expressing enhanced green fluorescent protein (eGFP) is described, in which human and mouse tumors marked with red fluorescent protein can be established in vivo, both at subcutaneous and orthotopic locations. Using light microscopy as well as multiphoton confocal microscopy techniques, we visualized in detail the intricate colocalization of tumor and host cells in situ. Moreover, using fluorescence-activated cell sorting (FACS), we were able to completely separate the host cells from the tumor cells, thus providing a system for detailed cellular and molecular analysis of tumor-host cell interactions. The fact that tumor and host cells can be reliably identified also allowed us to detect double-positive cells, possibly arising from cell fusion events or horizontal gene transfer. Similarly, the model can be applied for the detection of circulating metastatic cells and for detailed studies on the vascular compartments within tumors, including vasculogenic mimicry. Thus, the model described should provide significant insight into how tumor cells communicate with their microenvironment. Full Text Article | | 18495755
 |
Chemotherapy targets the hair-follicle vascular network but not the stem cells.
Amoh, Y; Li, L; Katsuoka, K; Hoffman, RM
The Journal of investigative dermatology
127
11-5
2007
Show Abstract
Chemotherapy-induced alopecia is a major problem in clinical oncology. Doxorubicin, a widely used cancer chemotherapy drug, induces disruption of the hair cycle and subsequent alopecia. We show in this report that doxorubicin causes disruption of the hair-follicle-associated blood vessel network resulting in a greatly reduced density of these blood vessels. Dystrophic hair follicles were also observed with abnormal melanogenesis in the mice treated with doxorubicin. Visualization of the effect of doxorubicin on hair-follicle angiogenesis was made possible by the use of transgenic mice in which green fluorescent protein was driven by regulatory elements of the nestin gene (ND-GFP). In these transgenic mice, the hair-follicle stem cells and the follicle structure as well as the blood vessels associated with the hair follicles express ND-GFP. The hair-follicle stem cells did not appear to be affected by doxorubicin, which may explain why hair regrows after chemotherapy. These results suggest that inhibition of hair-follicle-associated angiogenesis by doxorubicin may be an important factor in hair-follicle dystrophy associated with chemotherapy-induced alopecia. The ND-GFP mouse model is thus useful for the study of the role of angiogenesis in the hair-follicle cycle and the effect of drugs on processes associated with chemotherapy-induced alopecia. | | 16841031
 |
The camptothecin derivative CPT-11 inhibits angiogenesis in a dual-color imageable orthotopic metastatic nude mouse model of human colon cancer.
Yong Ji, Katsuhiro Hayashi, Yasuyuki Amoh, Kazuhiko Tsuji, Kensuke Yamauchi, Norio Yamamoto, Hiroyuki Tsuchiya, Katsuro Tomita, Michael Bouvet, Robert M Hoffman
Anticancer research
27
713-8
2007
Show Abstract
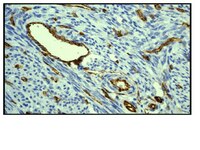
Recent studies have shown the expression of a stem cell marker protein, nestin, in nascent blood vessels in nestin-driven green fluorescent protein (ND-GFP) transgenic nude mice. In the present study, we visualized tumor angiogenesis and evaluated the antiangiogenic efficacy of CPT-11 in ND-GFP nude mice using dual-color fluorescence imaging. We orthotopically implanted ND-GFP nude mice with the human cancer cell line HCT-116 expressing red fluorescent protein (RFP). The mice were treated with CPT-11 at 40 mg/kg on days 7, 10, 14. Tumor angiogenesis was imaged and visualized by dual-color fluorescence imaging on day 17, three days after the last CPT-11 treatment. Tumor volume and the mean nascent blood vessel density were determined and compared to the control mice. The growing tumor had high expressions of nestin in the nascent blood vessels. The nascent blood vessels showed co-localization of the endothelial-cell-specific marker CD-31 under immunohistochemical staining. The nascent blood vessels were highly visible and their density was determined. ND-GFP nude mice that were administered CPT-11 showed significant reduction in the mean nascent blood vessel density and tumor volume. The dual-color model of ND-GFP transgenic nude mice orthotopically implanted with HCT-116 expressing RFP proved to be effective in visualizing and quantitating tumor growth and tumor angiogenesis. The results showed that CPT-11 is an effective inhibitor of angiogenesis and provided strong implications for wider clinical application of CPT-11 for colon cancer. | | 17465193
 |
Dual-color imaging of nascent blood vessels vascularizing pancreatic cancer in an orthotopic model demonstrates antiangiogenesis efficacy of gemcitabine.
Yasuyuki Amoh, Lingna Li, Kazuhiko Tsuji, A R Moossa, Kensei Katsuoka, Robert M Hoffman, Michael Bouvet
The Journal of surgical research
132
164-9
2006
Show Abstract
BACKGROUND: The stem cell marker nestin recently has been shown to be expressed in nascent blood vessels in nestin-driven green fluorescent protein (ND-GFP) transgenic nude mice. MATERIALS AND METHODS: In the present study, we visualized by dual-color fluorescence imaging tumor angiogenesis in the ND-GFP transgenic nude mice after orthotopic transplantation of the MIA PaCa-2 human pancreatic cancer line expressing red fluorescent protein. Mice were treated with gemcitabine at 150 mg/kg/dose on days 3, 6, 10, and 13 after tumor implantation. At day 14, mice were sacrificed and mean nascent blood vessel density and tumor volume were calculated and compared to control mice. RESULTS: Nestin was highly expressed in proliferating endothelial cells and nascent blood vessels in the growing tumor. Results of immunohistochemical staining showed that CD31 co-localized in ND-GFP-expressing nascent blood vessels. The density of nascent blood vessels in the tumor was readily quantitated. Gemcitabine significantly decreased the mean nascent blood vessel density in the tumor as well as decreased tumor volume. CONCLUSION: The dual-color model of the ND-GFP nude mouse orthotopically implanted with RFP-expressing pancreatic tumor cells enabled the simultaneous visualization and quantitation of tumor angiogenesis and tumor volume. These results demonstrated for the first time that gemcitabine is an inhibitor of angiogenesis as well as tumor growth in pancreatic cancer. The results have important implications for the clinical application of gemcitabine in this disease. | | 16500746
 |