Mechanoinduction of lymph vessel expansion.
Planas-Paz, L; Strilić, B; Goedecke, A; Breier, G; Fässler, R; Lammert, E
The EMBO journal
31
788-804
2012
Show Abstract
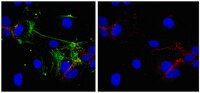
In the mammalian embryo, few mechanical signals have been identified to influence organ development and function. Here, we report that an increase in the volume of interstitial or extracellular fluid mechanically induces growth of an organ system, that is, the lymphatic vasculature. We first demonstrate that lymph vessel expansion in the developing mouse embryo correlates with a peak in interstitial fluid pressure and lymphatic endothelial cell (LEC) elongation. In 'loss-of-fluid' experiments, we then show that aspiration of interstitial fluid reduces the length of LECs, decreases tyrosine phosphorylation of vascular endothelial growth factor receptor-3 (VEGFR3), and inhibits LEC proliferation. Conversely, in 'gain-of-fluid' experiments, increasing the amount of interstitial fluid elongates the LECs, and increases both VEGFR3 phosphorylation and LEC proliferation. Finally, we provide genetic evidence that β1 integrins are required for the proliferative response of LECs to both fluid accumulation and cell stretching and, therefore, are necessary for lymphatic vessel expansion and fluid drainage. Thus, we propose a new and physiologically relevant mode of VEGFR3 activation, which is based on mechanotransduction and is essential for normal development and fluid homeostasis in a mammalian embryo. | 22157817
 |
In vivo optical molecular imaging of vascular endothelial growth factor for monitoring cancer treatment.
Chang, SK; Rizvi, I; Solban, N; Hasan, T
Clinical cancer research : an official journal of the American Association for Cancer Research
14
4146-53
2008
Show Abstract
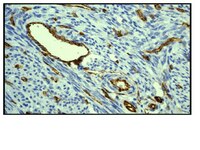
Vascular endothelial growth factor (VEGF) expression is a critical component in tumor growth and metastasis. Capabilities to monitor VEGF expression in vivo can potentially serve as a useful tool for diagnosis, prognosis, treatment planning, monitoring, and research. Here, we present the first report of in vivo hyperspectral molecular imaging strategy capable of monitoring treatment-induced changes in VEGF expression.VEGF was targeted with an anti-VEGF antibody conjugated with a fluorescent dye and was imaged in vivo using a hyperspectral imaging system. The strategy was validated by quantitatively monitoring VEGF levels in three different tumors as well as following photodynamic treatment. Specificity of the molecular imaging strategy was tested using in vivo competition experiments and mathematically using a quantitative pharmacokinetic model.The molecular imaging strategy successfully imaged VEGF levels quantitatively in three different tumors and showed concordance with results from standard ELISA. Changes in tumoral VEGF concentration following photodynamic treatment and Avastin treatment were shown. Immunohistochemistry shows that (a) the VEGF-specific contrast agent labels both proteoglycan-bound and unbound VEGF in the extracellular space and (b) the bound VEGF is released from the extracellular matrix in response to photodynamic therapy. In vivo competition experiments and quantitative pharmacokinetic model-based analysis confirmed the high specificity of the imaging strategy.This first report of in vivo quantitative optical molecular imaging-based monitoring of a secreted cytokine in tumors may have implications in providing tools for mechanistic investigations as well as for improved treatment design and merits further investigation. | 18593993
 |