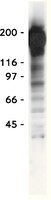
12/15-Lipoxygenase mediates high-fat diet-induced endothelial tight junction disruption and monocyte transmigration: a new role for 15(S)-hydroxyeicosatetraenoic acid in endothelial cell dysfunction.
Kundumani-Sridharan, V; Dyukova, E; Hansen, DE; Rao, GN
The Journal of biological chemistry
288
15830-42
2013
Show Abstract
A convincing body of evidence suggests that 12/15-lipoxygenase (12/15-LO) plays a role in atherosclerosis. However, the mechanisms of its involvement in the pathogenesis of this disease are not clear. Therefore, the purpose of this study is to understand the mechanisms by which 12/15-LO mediates endothelial dysfunction. 15(S)-Hydroxyeicosatetraenoic acid (15(S)-HETE), the major 12/15-LO metabolite of arachidonic acid (AA), induced endothelial barrier permeability via Src and Pyk2-dependent zonula occluden (ZO)-2 tyrosine phosphorylation and its dissociation from the tight junction complexes. 15(S)-HETE also stimulated macrophage adhesion to the endothelial monolayer in Src and Pyk2-dependent manner. Ex vivo studies revealed that exposure of arteries from WT mice to AA or 15(S)-HETE led to Src-Pyk2-dependent ZO-2 tyrosine phosphorylation, tight junction disruption, and macrophage adhesion, whereas the arteries from 12/15-LO knock-out mice are protected from these effects of AA. Feeding WT mice with a high-fat diet induced the expression of 12/15-LO in the arteries leading to tight junction disruption and macrophage adhesion and deletion of the 12/15-LO gene disallowed these effects. Thus, the findings of this study provide the first evidence of the role of 12/15-LO and its AA metabolite, 15(S)-HETE, in high-fat diet-induced endothelial tight junction disruption and macrophage adhesion, the crucial events underlying the pathogenesis of atherosclerosis. | | 23589307
 |
Both Kdr and Flt1 play a vital role in hypoxia-induced Src-PLD1-PKCγ-cPLA(2) activation and retinal neovascularization.
Singh, NK; Hansen, DE; Kundumani-Sridharan, V; Rao, GN
Blood
121
1911-23
2013
Show Abstract
To understand the mechanisms of Src-PLD1-PKCγ-cPLA2 activation by vascular endothelial growth factor A (VEGFA), we studied the role of Kdr and Flt1. VEGFA, while having no effect on Flt1 phosphorylation, induced Kdr phosphorylation in human retinal microvascular endothelial cells (HRMVECs). Depletion of Kdr attenuated VEGFA-induced Src-PLD1-PKCγ-cPLA2 activation. Regardless of its phosphorylation state, downregulation of Flt1 also inhibited VEGFA-induced Src-PLD1-PKCγ-cPLA2 activation, but only modestly. In line with these findings, depletion of either Kdr or Flt1 suppressed VEGFA-induced DNA synthesis, migration, and tube formation, albeit more robustly with Kdr downregulation. Hypoxia induced tyrosine phosphorylation of Kdr and Flt1 in mouse retina, and depletion of Kdr or Flt1 blocked hypoxia-induced Src-PLD1-PKCγ-cPLA2 activation and retinal neovascularization. VEGFB induced Flt1 tyrosine phosphorylation and Src-PLD1-PKCγ-cPLA2 activation in HRMVECs. Hypoxia induced VEGFA and VEGFB expression in retina, and inhibition of their expression blocked hypoxia-induced Kdr and Flt1 activation, respectively. Furthermore, depletion of VEGFA or VEGFB attenuated hypoxia-induced Src-PLD1-PKCγ-cPLA2 activation and retinal neovascularization. These findings suggest that although VEGFA, through Kdr and Flt1, appears to be the major modulator of Src-PLD1-PKCγ-cPLA2 signaling in HRMVECs, facilitating their angiogenic events in vitro, both VEGFA and VEGFB mediate hypoxia-induced Src-PLD1-PKCγ-cPLA2 activation and retinal neovascularization via activation of Kdr and Flt1, respectively. | | 23319572
 |
Inhibition of Grb2 expression demonstrates an important role in BCR-ABL-mediated MAPK activation and transformation of primary human hematopoietic cells.
Modi, H; Li, L; Chu, S; Rossi, J; Yee, JK; Bhatia, R
Leukemia
25
305-12
2011
Show Abstract
Chronic myeloid leukemia (CML) results from the expression of the BCR/ABL oncogene in a primitive hematopoietic cell. However, BCR/ABL-activated signaling mechanisms are dependent on the cellular context in which it is expressed, and mechanisms underlying primitive human hematopoietic cell transformation by BCR-ABL are not well understood. Our previous studies have shown that BCR/ABL-Y177 has an essential role in Ras activation and human hematopoietic progenitor transformation in CML. The adapter protein growth factor receptor-binding protein-2 (Grb2) can bind phosphorylated BCR/ABL-Y177, induce Grb2-SoS complex formation and activate Ras signaling. We investigated the role of Grb2 in CML progenitor transformation by cotransducing human CD34+ cells with lentivirus vectors expressing short hairpin RNA to Grb2 and retrovirus vectors expressing BCR/ABL. We show that Grb2 knockdown significantly inhibits proliferation and survival of BCR-ABL-expressing CD34+ cells, but not control CD34+ cells. Grb2 knockdown reduced mitogen-activated protein kinase (MAPK) activity in BCR-ABL-expressing hematopoietic cells. We conclude that inhibition of Grb2 expression demonstrates an important role in BCR-ABL-mediated MAPK activation and transformation of primary human hematopoietic cells.These results support further investigation of downstream effectors of Grb2-mediated signals and targeting of Grb2 interactions in the treatment of CML. | Western Blotting | 21072043
 |
Cytosolic lysine residues enhance anterograde transport and activation of the erythropoietin receptor.
Liron Yosha,Orly Ravid,Nathalie Ben-Califa,Drorit Neumann
The Biochemical journal
435
2011
Show Abstract
Lysine residues are key residues in many cellular processes, in part due to their ability to accept a wide variety of post-translational modifications. In the present study, we identify the EPO-R [EPO (erythropoietin) receptor] cytosolic lysine residues as enhancers of receptor function. EPO-R drives survival, proliferation and differentiation of erythroid progenitor cells via binding of its ligand EPO. We mutated the five EPO-R cytosolic lysine residues to arginine residues (5KR EPO-R), eliminating putative lysine-dependent modifications. Overexpressed 5KR EPO-R displayed impaired ubiquitination and improved stability compared with wt (wild-type) EPO-R. Unexpectedly, fusion proteins consisting of VSVGtsO45 (vesicular stomatitis virus glycoprotein temperature-sensitive folding mutant) with wt or 5KR EPO-R cytosolic domains demonstrated delayed glycan maturation kinetics upon substitution of the lysine residues. Moreover, VSVG-wt EPO-R, but not VSVG-5KR EPO-R, displayed endoplasmic reticulum-associated ubiquitination. Despite similar cell-surface EPO-binding levels of both receptors and the lack of EPO-induced ubiquitination by 5KR EPO-R, the lysine-less mutant produced weaker receptor activation and signalling than the wt receptor. We thus propose that EPO-R cytosolic lysine residues enhance receptor function, most probably through ubiquitination and/or other post-translational modifications. | | 21291419
 |
15-Lipoxygenase-1-enhanced Src-Janus kinase 2-signal transducer and activator of transcription 3 stimulation and monocyte chemoattractant protein-1 expression require redox-sensitive activation of epidermal growth factor receptor in vascular wall remodeling.
Singh, NK; Wang, D; Kundumani-Sridharan, V; Van Quyen, D; Niu, J; Rao, GN
The Journal of biological chemistry
286
22478-88
2011
Show Abstract
To understand the mechanisms by which 15(S)-hydroxyeicosatetraenoic acid (15(S)-HETE) activates signal transducer and activator of transcription 3 (STAT3), we studied the role of epidermal growth factor receptor (EGFR). 15(S)-HETE stimulated tyrosine phosphorylation of EGFR in a time-dependent manner in vascular smooth muscle cells (VSMCs). Interference with EGFR activation blocked 15(S)-HETE-induced Src and STAT3 tyrosine phosphorylation, monocyte chemoattractant protein-1 (MCP-1) expression and VSMC migration. 15(S)-HETE also induced tyrosine phosphorylation of Janus kinase 2 (Jak2) in VSMCs, and its inhibition substantially reduced STAT3 phosphorylation, MCP-1 expression, and VSMC migration. In addition, Src formed a complex with EGFR and Jak2, and its inhibition completely blocked Jak2 and STAT3 phosphorylation, MCP-1 expression, and VSMC migration. 15(S)-HETE induced the production of H(2)O(2) via an NADPH oxidase-dependent manner and its scavengers, N-acetyl cysteine (NAC) and catalase suppressed 15(S)-HETE-stimulated EGFR, Src, Jak2, and STAT3 phosphorylation and MCP-1 expression. Balloon injury (BI) induced EGFR, Src, Jak2, and STAT3 phosphorylation, and inhibition of these signaling molecules attenuated BI-induced MCP-1 expression and smooth muscle cell migration from the medial to the luminal surface resulting in reduced neointima formation. In addition, inhibition of EGFR blocked BI-induced Src, Jak2, and STAT3 phosphorylation. Similarly, interference with Src activation suppressed BI-induced Jak2 and STAT3 phosphorylation. Furthermore, adenovirus-mediated expression of dnJak2 also blocked BI-induced STAT3 phosphorylation. Consistent with the effects of 15(S)-HETE on the activation of EGFR-Src-Jak2-STAT3 signaling in VSMCs in vitro, adenovirus-mediated expression of 15-lipoxygenase 1 (15-Lox1) enhanced BI-induced EGFR, Src, Jak2, and STAT3 phosphorylation leading to enhanced MCP-1 expression in vivo. Blockade of Src or Jak2 suppressed BI-induced 15-Lox1-enhanced STAT3 phosphorylation, MCP-1 expression, and neointima formation. In addition, whereas dominant negative Src blocked BI-induced 15-Lox1-enhanced Jak2 phosphorylation, dnJak2 had no effect on Src phosphorylation. Together, these observations demonstrate for the first time that the 15-Lox1-15(S)-HETE axis activates EGFR via redox-sensitive manner, which in turn mediates Src-Jak2-STAT3-dependent MCP-1 expression leading to vascular wall remodeling. | | 21536676
 |
Motesanib inhibits Kit mutations associated with gastrointestinal stromal tumors.
Caenepeel, S; Renshaw-Gegg, L; Baher, A; Bush, TL; Baron, W; Juan, T; Manoukian, R; Tasker, AS; Polverino, A; Hughes, PE
Journal of experimental & clinical cancer research : CR
29
96
2010
Show Abstract
Activating mutations in Kit receptor tyrosine kinase or the related platelet-derived growth factor receptor (PDGFR) play an important role in the pathogenesis of gastrointestinal stromal tumors (GIST).This study investigated the activity of motesanib, an inhibitor of vascular endothelial growth factor receptors (VEGFR) 1, 2, and 3; PDGFR; and Kit, against primary activating Kit mutants and mutants associated with secondary resistance to imatinib. Single- and double-mutant isoforms of Kit were evaluated for their sensitivity to motesanib or imatinib in autophosphorylation assays and in Ba/F3 cell proliferation assays.Motesanib inhibited Kit autophosphorylation in CHO cell lines expressing primary activating mutations in exon 9 (AYins503-504, IC50 = 18 nM) and exon 11 (V560 D, IC50 = 5 nM; Delta552-559, IC50 = 1 nM). Motesanib also demonstrated activity against kinase domain mutations conferring imatinib resistance (V560D/V654A, IC50 = 77 nM; V560D/T670I, IC50 = 277 nM; Y823 D, IC50 = 64 nM) but failed to inhibit the imatinib-resistant D816V mutant (IC50 greater than 3000 nM). Motesanib suppressed the proliferation of Ba/F3 cells expressing Kit mutants with IC50 values in good agreement with those observed in the autophosphorylation assays.In conclusion, our data suggest that motesanib possesses inhibitory activity against primary Kit mutations and some imatinib-resistant secondary mutations. Full Text Article | | 20633291
 |
Interplay between the heterotrimeric G-protein subunits Galphaq and Galphai2 sets the threshold for chemotaxis and TCR activation.
Ngai, J; Inngjerdingen, M; Berge, T; Taskén, K
BMC immunology
10
27
2009
Show Abstract
TCR and CXCR4-mediated signaling appears to be reciprocally regulated pathways. TCR activation dampens the chemotactic response towards the CXCR4 ligand CXCL12, while T cells exposed to CXCL12 are less prone to subsequent TCR-activation. The heterotrimeric G proteins Galphaq and Galphai2 have been implicated in CXCR4-signaling and we have recently also reported the possible involvement of Galphaq in TCR-dependent activation of Lck (Ngai et al., Eur. J. Immunol., 2008, 38: 32083218). Here we examined the role of Galphaq in migration and TCR activation.Pre-treatment of T cells with CXCL12 led to significantly reduced Lck Y394 phosphorylation upon TCR triggering indicating heterologous desensitization. We show that knockdown of Galphaq significantly enhanced basal migration in T cells and reduced CXCL12-induced SHP-1 phosphorylation whereas Galphai2 knockdown inhibited CXCL12-induced migration.Our data suggest that Galphai2 confers migration signals in the presence of CXCL12 whereas Galphaq exerts a tonic inhibition on both basal and stimulated migrational responses. This is compatible with the notion that the level of Galphaq activation contributes to determining the commitment of the T cell either to migration or activation through the TCR. Full Text Article | | 19426503
 |