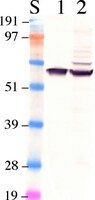
Translational read-through of the RP2 Arg120stop mutation in patient iPSC-derived retinal pigment epithelium cells.
Schwarz, N; Carr, AJ; Lane, A; Moeller, F; Chen, LL; Aguilà, M; Nommiste, B; Muthiah, MN; Kanuga, N; Wolfrum, U; Nagel-Wolfrum, K; da Cruz, L; Coffey, PJ; Cheetham, ME; Hardcastle, AJ
Human molecular genetics
24
972-86
2015
Show Abstract
Mutations in the RP2 gene lead to a severe form of X-linked retinitis pigmentosa. RP2 patients frequently present with nonsense mutations and no treatments are currently available to restore RP2 function. In this study, we reprogrammed fibroblasts from an RP2 patient carrying the nonsense mutation c.519Cgreater than T (p.R120X) into induced pluripotent stem cells (iPSC), and differentiated these cells into retinal pigment epithelial cells (RPE) to study the mechanisms of disease and test potential therapies. RP2 protein was undetectable in the RP2 R120X patient cells, suggesting a disease mechanism caused by complete lack of RP2 protein. The RP2 patient fibroblasts and iPSC-derived RPE cells showed phenotypic defects in IFT20 localization, Golgi cohesion and Gβ1 trafficking. These phenotypes were corrected by over-expressing GFP-tagged RP2. Using the translational read-through inducing drugs (TRIDs) G418 and PTC124 (Ataluren), we were able to restore up to 20% of endogenous, full-length RP2 protein in R120X cells. This level of restored RP2 was sufficient to reverse the cellular phenotypic defects observed in both the R120X patient fibroblasts and iPSC-RPE cells. This is the first proof-of-concept study to demonstrate successful read-through and restoration of RP2 function for the R120X nonsense mutation. The ability of the restored RP2 protein level to reverse the observed cellular phenotypes in cells lacking RP2 indicates that translational read-through could be clinically beneficial for patients. | | 25292197
 |
Retinoid uptake, processing, and secretion in human iPS-RPE support the visual cycle.
Muñiz, A; Greene, WA; Plamper, ML; Choi, JH; Johnson, AJ; Tsin, AT; Wang, HC
Investigative ophthalmology & visual science
55
198-209
2014
Show Abstract
Retinal pigmented epithelium derived from human induced pluripotent stem (iPS) cells (iPS-RPE) may be a source of cells for transplantation. For this reason, it is essential to determine the functional competence of iPS-RPE. One key role of the RPE is uptake and processing of retinoids via the visual cycle. The purpose of this study is to investigate the expression of visual cycle proteins and the functional ability of the visual cycle in iPS-RPE.iPS-RPE was derived from human iPS cells. Immunocytochemistry, RT-PCR, and Western blot analysis were used to detect expression of RPE genes lecithin-retinol acyl transferase (LRAT), RPE65, cellular retinaldehyde-binding protein (CRALBP), and pigment epithelium-derived factor (PEDF). All-trans retinol was delivered to cultured cells or whole cell homogenate to assess the ability of the iPS-RPE to process retinoids.Cultured iPS-RPE expresses visual cycle genes LRAT, CRALBP, and RPE65. After incubation with all-trans retinol, iPS-RPE synthesized up to 2942 ± 551 pmol/mg protein all-trans retinyl esters. Inhibition of LRAT with N-ethylmaleimide (NEM) prevented retinyl ester synthesis. Significantly, after incubation with all-trans retinol, iPS-RPE released 188 ± 88 pmol/mg protein 11-cis retinaldehyde into the culture media.iPS-RPE develops classic RPE characteristics and maintains expression of visual cycle proteins. The results of this study confirm that iPS-RPE possesses the machinery to process retinoids for support of visual pigment regeneration. Inhibition of all-trans retinyl ester accumulation by NEM confirms LRAT is active in iPS-RPE. Finally, the detection of 11-cis retinaldehyde in the culture medium demonstrates the cells' ability to process retinoids through the visual cycle. This study demonstrates expression of key visual cycle machinery and complete visual cycle activity in iPS-RPE. | | 24255038
 |
Small molecule mediated proliferation of primary retinal pigment epithelial cells.
Swoboda, JG; Elliott, J; Deshmukh, V; de Lichtervelde, L; Shen, W; Tremblay, MS; Peters, EC; Cho, CY; Lu, B; Girman, S; Wang, S; Schultz, PG
ACS chemical biology
8
1407-11
2013
Show Abstract
Retinal pigment epithelial (RPE) cells form a monolayer adjacent to the retina and play a critical role in the visual light cycle. Degeneration of RPE cells results in retinal disorders such as age-related macular degeneration. Cell transplant strategies have potential therapeutic value for such disorders; however, risks associated with an inadequate supply of donor cells limit their therapeutic success. The identification of factors that proliferate RPE cells ex vivo could provide a renewable source of cells for transplantation. Here, we report that a small molecule (WS3) can reversibly proliferate primary RPE cells isolated from fetal and adult human donors. Following withdrawal of WS3, RPE cells differentiate into a functional monolayer, as exhibited by their expression of mature RPE genes and phagocytosis of photoreceptor outer segments. Furthermore, chemically expanded RPE cells preserve vision when transplanted into dystrophic Royal College of Surgeons (RCS) rats, a well-established model of retinal degeneration. | | 23621521
 |
Attenuation of choroidal neovascularization by β(2)-adrenoreceptor antagonism.
Lavine, JA; Sang, Y; Wang, S; Ip, MS; Sheibani, N
JAMA ophthalmology
131
376-82
2013
Show Abstract
To determine whether β-adrenergic blockade inhibits choroidal neovascularization (CNV) in a mouse model of laser-induced CNV and to investigate the mechanism by which β-adrenoreceptor antagonism blunts CNV.Mice were subjected to laser burns, inducing CNV, and were treated with daily intraperitoneal injections of propranolol hydrochloride. Neovascularization was measured on choroidal-scleral flat mounts using intercellular adhesion molecule 2 immunofluorescence staining. The effect of β-adrenoreceptor signaling on expression of vascular endothelial growth factor (VEGF) was investigated using primary mouse choroidal endothelial cells (ChECs) and retinal pigment epithelial (RPE) cells. These cells were incubated with β-adrenoreceptor agonists and/or antagonists and assayed for Vegf messenger RNA and protein levels.University of Wisconsin School of Medicine and Public Health.Wild-type 6-week-old female C57BL/6j mice.Inhibition of CNV after propranolol treatment and Vegf messenger RNA and protein expression after treatment with β-adrenoreceptor agonists and antagonists.Propranolol-treated mice demonstrated a 50% reduction in laser-induced CNV. Treatment with norepinephrine bitartrate stimulated Vegf messenger RNA expression and protein secretion in ChECs and RPE cells. This effect was blocked by β2-adrenoreceptor antagonism and mimicked by β2-adrenoreceptor agonists.Attenuation of CNV is achieved by β-adrenergic blockade. The β2-adrenoreceptors regulate VEGF expression in ChECs and RPE cells.Antagonists of β-adrenoreceptors are safe and well tolerated in patients with glaucoma and cardiovascular disease. Thus, blockade of β-adrenoreceptors may provide a new avenue to inhibit VEGF expression in CNV. | | 23303344
 |
Patient-specific iPSC-derived photoreceptor precursor cells as a means to investigate retinitis pigmentosa.
Tucker, BA; Mullins, RF; Streb, LM; Anfinson, K; Eyestone, ME; Kaalberg, E; Riker, MJ; Drack, AV; Braun, TA; Stone, EM
eLife
2
e00824
2013
Show Abstract
Next-generation and Sanger sequencing were combined to identify disease-causing USH2A mutations in an adult patient with autosomal recessive RP. Induced pluripotent stem cells (iPSCs), generated from the patient's keratinocytes, were differentiated into multi-layer eyecup-like structures with features of human retinal precursor cells. The inner layer of the eyecups contained photoreceptor precursor cells that expressed photoreceptor markers and exhibited axonemes and basal bodies characteristic of outer segments. Analysis of the USH2A transcripts of these cells revealed that one of the patient's mutations causes exonification of intron 40, a translation frameshift and a premature stop codon. Western blotting revealed upregulation of GRP78 and GRP94, suggesting that the patient's other USH2A variant (Arg4192His) causes disease through protein misfolding and ER stress. Transplantation into 4-day-old immunodeficient Crb1 (-/-) mice resulted in the formation of morphologically and immunohistochemically recognizable photoreceptor cells, suggesting that the mutations in this patient act via post-developmental photoreceptor degeneration. DOI:http://dx.doi.org/10.7554/eLife.00824.001. | | 23991284
 |
c-Met modulates RPE migratory response to laser-induced retinal injury.
Kasaoka, M; Ma, J; Lashkari, K
PloS one
7
e40771
2012
Show Abstract
Retinal laser injuries are often associated with aberrant migration of the retinal pigment epithelium (RPE), which can cause expansion of the scar beyond the confines of the original laser burn. In this study, we devised a novel method of laser-induced injury to the RPE layer in mouse models and began to dissect the mechanisms associated with pathogenesis and progression of laser-induced RPE injury. We have hypothesized that the proto-oncogene receptor, c-Met, is intimately involved with migration of RPE cells, and may be an early responder to injury. Using transgenic mouse models, we show that constitutive activation of c-Met induces more robust RPE migration into the outer retina of laser-injured eyes, while abrogation of the receptor using a cre-lox method reduces these responses. We also demonstrate that retinal laser injury increases expression of both HGF and c-Met, and activation of c-Met after injury is correlated with RPE cell migration. RPE migration may be responsible for clinically significant anatomic changes observed after laser injury. Abrogation of c-Met activity may be a therapeutic target to minimize retinal damage from aberrant RPE cell migration. | Immunohistochemistry | 22808260
 |
MEK-ERK signaling in adult newt retinal pigment epithelium cells is strengthened immediately after surgical induction of retinal regeneration.
Aki Mizuno,Hirofumi Yasumuro,Taro Yoshikawa,Wataru Inami,Chikafumi Chiba
Neuroscience letters
523
2012
Show Abstract
Adult newt retinal pigment epithelium (RPE) cells are mitotically quiescent in the physiological condition, but upon a traumatic injury of the neural retina (NR) they re-enter the cell-cycle and eventually regenerate the missing NR. Here, to understand the mechanism underlying the cell-cycle re-entry of RPE cells following NR injury, we first investigated changes in MEK-ERK signaling activity in RPE cells upon removal of the NR (retinectomy) from the eye of living animals, and found that ERK-mediated signaling activity is elevated quickly (in 30min) upon retinectomy. In addition, we found, in in vitro analyses, that immediate early activation of MEK-ERK signaling may occur in RPE cells upon NR injury, intensifying the MEK-ERK signaling itself through up-regulation of the expression of constituent molecules in the pathway, and that 1-h blockade of such early MEK-ERK signaling interferes with the cell-cycle re-entry, which occurs 5-10 days later. Together, these results provide us with insight that elevation of MEK-ERK signaling activity upon NR injury may be a key process for mitotically quiescent RPE cells to re-enter the cell-cycle, leading to retinal regeneration. | | 22743657
 |
FATP1 inhibits 11-cis retinol formation via interaction with the visual cycle retinoid isomerase RPE65 and LRAT.
Guignard TJ, Jin M, Pequignot MO, Li S, Chassigneux Y, Chekroud K, Guillou L, Richard E, Hamel CP, Brabet P
J Biol Chem
2010
Show Abstract
The isomerization of all-trans retinol (vitamin A) to 11-cis retinol in the retinal pigment epithelium (RPE) is a key step in the visual process for the regeneration of the visual pigment chromophore, 11-cis retinal. LRAT and RPE65 are recognized as the minimal isomerase catalytic components. However, regulators of this rate-limiting step are not fully identified and could account for the phenotypic variability associated with inherited retinal degeneration (RD) caused by mutations in the RPE65 gene. To identify new RPE65 partners, we screened a porcine RPE mRNA library using a yeast two-hybrid assay with full-length human RPE65. One identified clone (here named FATP1c), containing cytosolic C-terminal sequence from the fatty acid transport protein 1 (FATP1 or SLC27A1, solute carrier family 27 member 1), was demonstrated to interact dose-dependently with the native RPE65 and with LRAT. Furthermore, these interacting proteins colocalize in the RPE. Cellular reconstitution of human interacting proteins shows that FATP1 markedly inhibits 11-cis retinol production by acting on the production of all-trans retinyl esters and the isomerase activity of RPE65. The identification of this new visual cycle inhibitory component in RPE may contribute to further understanding of retinal pathogenesis. | | 20356843
 |
Musashi-1, an RNA-binding protein, is indispensable for survival of photoreceptors.
Kanako Susaki,Jun Kaneko,Yuka Yamano,Kenta Nakamura,Wataru Inami,Taro Yoshikawa,Yoko Ozawa,Shinsuke Shibata,Osamu Matsuzaki,Hideyuki Okano,Chikafumi Chiba
Experimental eye research
88
2009
Show Abstract
Musashi-1 (Msi1), an RNA-binding protein (RBP), has been postulated to play important roles in the maintenance of the stem-cell state, differentiation, and tumorigenesis. However, the expression and function of Msi1 in differentiated cells remain obscure. Here we show that Msi1 is expressed in mature photoreceptors and retinal pigment epithelium (RPE) cells, and is indispensable for the survival of photoreceptors. We found in the adult newt eye that Msi1 is expressed in all photoreceptors and RPE cells as well as in the retinal stem/progenitor cells in the ciliary marginal zone (CMZ). We found in the analyses of the newt normal and regenerating retinas that the expression profiles of the Msi1 transcripts and protein isoforms in the photoreceptors are different from those in the retinal stem/progenitor cells. Furthermore, we found that all photoreceptors and RPE cells of the adult mice also express Msi1, and that Msi1 knockout (Msi1-KO) results in degeneration of photoreceptors and a lack of a visual cycle protein RPE65 in the microvilli of RPE cells. Taken together, our current results demonstrate that the expression of Msi1 in mature photoreceptors and RPE cells is evolutionarily conserved, and that Msi1 bears essential functions for vision. Considering such an Msi1-KO phenotype in the retina, it is now reasonable to address whether defects of the Msi1 functions are responsible for inherited retinal diseases. Studying the regulation of Msi1 and the target RNAs of Msi1 in photoreceptors and RPE cells might contribute to fundamental and clinical studies of retinal degeneration. | | 18662689
 |
Visual cycle protein RPE65 persists in new retinal cells during retinal regeneration of adult newt.
Chikafumi Chiba, Akika Hoshino, Kenta Nakamura, Kanako Susaki, Yuka Yamano, Yuko Kaneko, Osamu Kuwata, Fumiaki Maruo, Takehiko Saito
The Journal of comparative neurology
495
391-407
2006
Show Abstract
Adult newts can regenerate their entire retina through transdifferentiation of the retinal pigment epithelium (RPE). The objective of this study was to redescribe the retina regeneration process by means of modern biological techniques. We report two different antibodies (RPE-No.112 and MAB5428) that recognize the newt homolog of RPE65, which is involved in the visual cycle and exclusively label the RPE cell-layer in the adult newt eye. We analyzed the process of retinal regeneration by immunohistochemistry and immunoblotting and propose that this process should be divided into nine stages. We found that the RPE65 protein is present in the RPE-derived new retinal rudiment at 14 days postoperative (po) and in the regenerating retinas at the 3-4 cell stage (19 days po). These observations suggest that certain characteristics of RPE cells overlap with those of retinal stem/progenitor cells during the period of transdifferentiation. However, RPE65 protein was not detected in either retinal stem/progenitor cells in the ciliary marginal zone (CMZ) of adult eyes or in neuroepithelium present during retina development, where it was first detected in differentiated RPE. Moreover, the gene expression of RPE65 was drastically downregulated in the early phase of transdifferentiation (by 10 days po), while those of Connexin43 and Pax-6, both expressed in regenerating retinas, were differently upregulated. These observations suggest that the RPE65 protein in the RPE-derived retinal rudiment may represent the remainder after protein degradation or discharge rather than newly synthesized protein. | | 16485283
 |