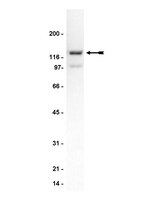
Dopaminergic dysregulation in prefrontal cortex of rhesus monkeys following cocaine self-administration.
McIntosh, S; Howell, L; Hemby, SE
Frontiers in psychiatry
4
88
2013
Show Abstract
Chronic cocaine administration regulates the expression of several proteins related to dopaminergic signaling and synaptic function in the mesocorticolimbic pathway, including the prefrontal cortex. Functional abnormalities in the prefrontal cortex are hypothesized to be due in part to the expression of proteins involved in dopamine signaling and plasticity. Adult male rhesus monkeys self-administered cocaine (i.v.) under limited (n = 4) and extended access conditions (n = 6). The abundance of surrogate markers of dopamine signaling and plasticity in the dorsolateral prefrontal cortex (DLPFC), orbitofrontal cortex (OFC), and anterior cingulate cortex (ACC) were examined: glycosylated and non-glycosylated forms of the dopamine transporter (efficiency of dopamine transport), tyrosine hydroxylase (TH; marker of dopamine synthesis) and phosphorylated TH at Serine 30 and 40 (markers of enzyme activity), extracellular signal-regulated kinase 1 and 2 (ERK1 and ERK 2), and phosphorylated ERK1 and ERK2 (phosphorylates TH Serine 31; markers of synaptic plasticity), and markers of synaptic integrity, spinophilin and post-synaptic density protein 95 (roles in dopamine signaling and response to cocaine). Extended cocaine access increased non-glycosylated and glycosylated DAT in DLPFC and OFC. While no differences in TH expression were observed between groups for any of the regions, extended access induced significant elevations in pTH(Ser31) in all regions. In addition, a slight but significant reduction in phosphorylated pTH(Ser40) was found in the DLPFC. Phosphorylated ERK2 was increased in all regions; however, pERK1 was decreased in ACC and OFC but increased in DLPFC. PSD-95 was increased in the OFC but not in DLPFC or ACC. Furthermore, extended cocaine self-administration elicited significant increases in spinophilin protein expression in all regions. Results from the study provide insight into the biochemical alterations occurring in primate prefrontal cortex. | | 23970867
 |
Identification and validation of novel spinophilin-associated proteins in rodent striatum using an enhanced ex vivo shotgun proteomics approach.
Baucum, AJ; Jalan-Sakrikar, N; Jiao, Y; Gustin, RM; Carmody, LC; Tabb, DL; Ham, AJ; Colbran, RJ
Molecular & cellular proteomics : MCP
9
1243-59
2010
Show Abstract
Spinophilin regulates excitatory postsynaptic function and morphology during development by virtue of its interactions with filamentous actin, protein phosphatase 1, and a plethora of additional signaling proteins. To provide insight into the roles of spinophilin in mature brain, we characterized the spinophilin interactome in subcellular fractions solubilized from adult rodent striatum by using a shotgun proteomics approach to identify proteins in spinophilin immune complexes. Initial analyses of samples generated using a mouse spinophilin antibody detected 23 proteins that were not present in an IgG control sample; however, 12 of these proteins were detected in complexes isolated from spinophilin knock-out tissue. A second screen using two different spinophilin antibodies and either knock-out or IgG controls identified a total of 125 proteins. The probability of each protein being specifically associated with spinophilin in each sample was calculated, and proteins were ranked according to a chi(2) analysis of the probabilities from analyses of multiple samples. Spinophilin and the known associated proteins neurabin and multiple isoforms of protein phosphatase 1 were specifically detected. Multiple, novel, spinophilin-associated proteins (myosin Va, calcium/calmodulin-dependent protein kinase II, neurofilament light polypeptide, postsynaptic density 95, alpha-actinin, and densin) were then shown to interact with GST fusion proteins containing fragments of spinophilin. Additional biochemical and transfected cell imaging studies showed that alpha-actinin and densin directly interact with residues 151-300 and 446-817, respectively, of spinophilin. Taken together, we have developed a multi-antibody, shotgun proteomics approach to characterize protein interactomes in native tissues, delineating the importance of knock-out tissue controls and providing novel insights into the nature and function of the spinophilin interactome in mature striatum. | | 20124353
 |
Age-related changes in Usp9x protein expression and DNA methylation in mouse brain.
Jun Xu
Brain research. Molecular brain research
140
17-24
2005
Show Abstract
Usp9x, a ubiquitin-specific protease implicated in synaptic development, was found to be more abundant in adult as compared to newborn mouse brain tissue. The Usp9x gene was less methylated in adults than in newborns in both the promoter and the protein coding region. Compared with newborns, the adult mouse brain also had lower levels of Dnmt1, the enzyme responsible for maintaining DNA methylation state. These age-associated changes in DNA methylation and ubiquitin system protein concentrations potentially contribute to developmental changes in brain structure and function. | | 16023255
 |
Phosphorylation-dependent regulation of ryanodine receptors: a novel role for leucine/isoleucine zippers.
Marx, S O, et al.
J. Cell Biol., 153: 699-708 (2001)
2001
Show Abstract
Ryanodine receptors (RyRs), intracellular calcium release channels required for cardiac and skeletal muscle contraction, are macromolecular complexes that include kinases and phosphatases. Phosphorylation/dephosphorylation plays a key role in regulating the function of many ion channels, including RyRs. However, the mechanism by which kinases and phosphatases are targeted to ion channels is not well understood. We have identified a novel mechanism involved in the formation of ion channel macromolecular complexes: kinase and phosphatase targeting proteins binding to ion channels via leucine/isoleucine zipper (LZ) motifs. Activation of kinases and phosphatases bound to RyR2 via LZs regulates phosphorylation of the channel, and disruption of kinase binding via LZ motifs prevents phosphorylation of RyR2. Elucidation of this new role for LZs in ion channel macromolecular complexes now permits: (a) rapid mapping of kinase and phosphatase targeting protein binding sites on ion channels; (b) predicting which kinases and phosphatases are likely to regulate a given ion channel; (c) rapid identification of novel kinase and phosphatase targeting proteins; and (d) tools for dissecting the role of kinases and phosphatases as modulators of ion channel function. | Immunoblotting (Western) | 11352932
 |
Neurabin-II/spinophilin. An actin filament-binding protein with one pdz domain localized at cadherin-based cell-cell adhesion sites.
Satoh, A, et al.
J. Biol. Chem., 273: 3470-5 (1998)
1998
Show Abstract
In a preceding paper, we reported a novel actin filament (F-actin)-binding protein, named neurabin, which was specifically expressed in neural tissue and implicated in neurite formation. We purified from rat brain another F-actin-binding protein, which had a domain organization similar to that of neurabin but was ubiquitously expressed, and named it neurabin-II. The original neurabin, renamed neurabin-I, had 1095 amino acids and a calculated Mr of 122,729, whereas neurabin-II had 817 amino acids and a calculated Mr of 89, 642. Both neurabin-I and -II had one F-actin-binding domain at the N-terminal region, one PDZ domain at the middle region, a domain known to interact with transmembrane proteins, and domains predicted to form coiled-coil structures at the C-terminal region. Both neurabin-I and -II bound along the sides of F-actin and showed F-actin-cross-linking activity. The subcellular distribution analysis indicated that neurabin-II was enriched at the postsynaptic density fraction in rat brain and the adherens junction fraction in rat liver. Immunofluorescence microscopic analysis revealed that neurabin-II was highly concentrated at the synapse in primary cultured rat hippocampal neurons and at the cadherin-based cell-cell adhesion sites in Madin-Darby canine kidney cells. Neurabin-II turned out to be the same as a recently reported protein phosphatase 1-binding protein named spinophilin. These results suggest that neurabin-II/spinophilin plays an important role in linking the actin cytoskeleton to the plasma membrane. | | 9452470
 |