MET receptor tyrosine kinase controls dendritic complexity, spine morphogenesis, and glutamatergic synapse maturation in the hippocampus.
Qiu, S; Lu, Z; Levitt, P
The Journal of neuroscience : the official journal of the Society for Neuroscience
34
16166-79
2014
Show Abstract
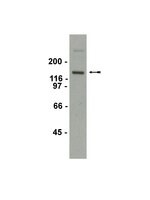
The MET receptor tyrosine kinase (RTK), implicated in risk for autism spectrum disorder (ASD) and in functional and structural circuit integrity in humans, is a temporally and spatially regulated receptor enriched in dorsal pallial-derived structures during mouse forebrain development. Here we report that loss or gain of function of MET in vitro or in vivo leads to changes, opposite in nature, in dendritic complexity, spine morphogenesis, and the timing of glutamatergic synapse maturation onto hippocampus CA1 neurons. Consistent with the morphological and biochemical changes, deletion of Met in mutant mice results in precocious maturation of excitatory synapse, as indicated by a reduction of the proportion of silent synapses, a faster GluN2A subunit switch, and an enhanced acquisition of AMPA receptors at synaptic sites. Thus, MET-mediated signaling appears to serve as a mechanism for controlling the timing of neuronal growth and functional maturation. These studies suggest that mistimed maturation of glutamatergic synapses leads to the aberrant neural circuits that may be associated with ASD risk. | Immunohistochemistry | 25471559
 |
Reduced metabotropic glutamate receptor 5 density in major depression determined by [(11)C]ABP688 PET and postmortem study.
Deschwanden, A; Karolewicz, B; Feyissa, AM; Treyer, V; Ametamey, SM; Johayem, A; Burger, C; Auberson, YP; Sovago, J; Stockmeier, CA; Buck, A; Hasler, G
The American journal of psychiatry
168
727-34
2011
Show Abstract
Clinical and preclinical evidence suggests a hyperactive glutamatergic system in clinical depression. Recently, the metabotropic glutamate receptor 5 (mGluR5) has been proposed as an attractive target for novel therapeutic approaches to depression. The goal of this study was to compare mGluR5 binding (in a positron emission tomography [PET] study) and mGluR5 protein expression (in a postmortem study) between individuals with major depressive disorder and psychiatrically healthy comparison subjects.Images of mGluR5 receptor binding were acquired using PET with [(11)C]ABP688, which binds to an allosteric site with high specificity, in 11 unmedicated individuals with major depression and 11 matched healthy comparison subjects. The amount of mGluR5 protein was investigated using Western blot in postmortem brain samples of 15 depressed individuals and 15 matched comparison subjects.The PET study revealed lower levels of regional mGluR5 binding in the prefrontal cortex, the cingulate cortex, the insula, the thalamus, and the hippocampus in the depression group relative to the comparison group. Severity of depression was negatively correlated with mGluR5 binding in the hippocampus. The postmortem study showed lower levels of mGluR5 protein expression in the prefrontal cortex (Brodmann's area 10) in the depression group relative to the comparison group, while prefrontal mGluR1 protein expression did not differ between groups.The lower levels of mGluR5 binding observed in the depression group are consonant with the lower levels of protein expression in brain tissue in the postmortem depression group. Thus, both studies suggest that basal or compensatory changes in excitatory neurotransmission play roles in the pathophysiology of major depression. | | 21498461
 |
Chronic corticosterone administration down-regulates metabotropic glutamate receptor 5 protein expression in the rat hippocampus.
Iyo, AH; Feyissa, AM; Chandran, A; Austin, MC; Regunathan, S; Karolewicz, B
Neuroscience
169
1567-74
2010
Show Abstract
Several lines of evidence suggest a dysfunctional glutamate system in major depressive disorder (MDD). Recently, we reported reduced levels of metabotropic glutamate receptor subtype 5 (mGluR5) in postmortem brains in MDD, however the neurobiological mechanisms that induce these abnormalities are unclear. In the present study, we examined the effect of chronic corticosterone (CORT) administration on the expression of mGluR5 protein and mRNA in the rat frontal cortex and hippocampus. Rats were injected with CORT (40 mg/kg s.c.) or vehicled once daily for 21 days. The expression of mGluR5 protein and mRNA was assessed by Western blotting and quantitative real-time PCR (qPCR). In addition, mGluR1 protein was measured in the same animals. The results revealed that while there was a significant reduction (-27%, P=0.0006) in mGluR5 protein expression in the hippocampus from CORT treated rats, mRNA levels were unchanged. Also unchanged were mGluR5 mRNA and protein levels in the frontal cortex and mGluR1 protein levels in both brain regions. Our findings provide the first evidence that chronic CORT exposure regulates the expression of mGluR5 and are in line with previous postmortem and imaging studies showing reduced mGluR5 in MDD. Our findings suggest that elevated levels of glucocorticoids may contribute to impairments in glutamate neurotransmission in MDD. Full Text Article | Western Blotting | 20600666
 |
Structure and function of glutamate receptor ion channels.
Mayer, Mark L and Armstrong, Neali
Annu. Rev. Physiol., 66: 161-81 (2004)
2004
Show Abstract
A vast number of proteins are involved in synaptic function. Many have been cloned and their functional role defined with varying degrees of success, but their number and complexity currently defy any molecular understanding of the physiology of synapses. A beacon of success in this medieval era of synaptic biology is an emerging understanding of the mechanisms underlying the activity of the neurotransmitter receptors for glutamate. Largely as a result of structural studies performed in the past three years we now have a mechanistic explanation for the activation of channel gating by agonists and partial agonists; the process of desensitization, and its block by allosteric modulators, is also mostly explained; and the basis of receptor subtype selectivity is emerging with clarity as more and more structures are solved. In the space of months we have gone from cartoons of postulated mechanisms to hard fact. It is anticipated that this level of understanding will emerge for other synaptic proteins in the coming decade. | | 14977400
 |
Glutamate and the glutamate receptor system: a target for drug action.
Bleich, Stefan, et al.
International journal of geriatric psychiatry, 18: S33-40 (2003)
2003
| | 12973748
 |
Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer.
Ango, F, et al.
Nature, 411: 962-5 (2001)
2001
| | 11418862
 |
The G-protein-coupled receptor kinase GRK4 mediates homologous desensitization of metabotropic glutamate receptor 1.
Sallese, M, et al.
FASEB J., 14: 2569-80 (2000)
2000
Show Abstract
G-protein-coupled receptor kinases (GRKs) are involved in the regulation of many G-protein-coupled receptors. As opposed to the other GRKs, such as rhodopsin kinase (GRK1) or beta-adrenergic receptor kinase (beta ARK, GRK2), no receptor substrate for GRK4 has been so far identified. Here we show that GRK4 is expressed in cerebellar Purkinje cells, where it regulates mGlu(1) metabotropic glutamate receptors, as indicated by the following: 1) When coexpressed in heterologous cells (HEK293), mGlu(1) receptor signaling was desensitized by GRK4 in an agonist-dependent manner (homologous desensitization). 2) In transfected HEK293 and in cultured Purkinje cells, the exposure to glutamate agonists induced internalization of the receptor and redistribution of GRK4. There was a substantial colocalization of the receptor and kinase both under basal condition and after internalization. 3) Kinase activity was necessary for desensitizing mGlu(1a) receptor and agonist-dependent phosphorylation of this receptor was also documented. 4) Antisense treatment of cultured Purkinje cells, which significantly reduced the levels of GRK4 expression, induced a marked modification of the mGlu(1)-mediated functional response, consistent with an impaired receptor desensitization. The critical role for GRK4 in regulating mGlu(1) receptors implicates a major involvement of this kinase in the physiology of Purkinje cell and in motor learning. | | 11099476
 |
G protein-coupled receptor kinase-mediated desensitization of metabotropic glutamate receptor 1A protects against cell death.
Dale, L B, et al.
J. Biol. Chem., 275: 38213-20 (2000)
2000
Show Abstract
Metabotropic glutamate receptors (mGluRs) constitute a unique subclass of G protein-coupled receptors (GPCRs) that bear little sequence homology to other members of the GPCR superfamily. The mGluR subtypes that are coupled to the hydrolysis of phosphoinositide contribute to both synaptic plasticity and glutamate-mediated excitotoxicity in neurons. In the present study, the expression of mGluR1a in HEK 293 cells led to agonist-independent cell death. Since G protein-coupled receptor kinases (GRKs) desensitize a diverse variety of GPCRs, we explored whether GRKs contributed to the regulation of both constitutive and agonist-stimulated mGluR1a activity and thereby may prevent mGluR1a-mediated excitotoxicity associated with mGluR1a overactivation. We find that the co-expression of mGluR1a with GRK2 and GRK5, but not GRK4 and GRK6, reduced both constitutive and agonist-stimulated mGluR1a activity. Agonist-stimulated mGluR1a phosphorylation was enhanced by the co-expression of GRK2 and was blocked by two different GRK2 dominant-negative mutants. Furthermore, GRK2-dependent mGluR1a desensitization protected against mGluR1a-mediated cell death, at least in part by blocking mGluR1a-stimulated apoptosis. Our data indicate that as with other members of the GPCR superfamily, a member of the structurally distinct mGluR family (mGluR1a) serves as a substrate for GRK-mediated phosphorylation and that GRK-dependent "feedback" modulation of mGluR1a responsiveness protects against pathophysiological mGluR1a signaling. | | 10982802
 |
Immunocytochemical localization of the mGluR1 alpha metabotropic glutamate receptor in the dorsal cochlear nucleus.
Wright, D D, et al.
J. Comp. Neurol., 364: 729-45 (1996)
1996
Show Abstract
We demonstrate that the metabotropic glutamate receptor mGluR1 alpha is enriched in two interneuron cell populations in the dorsal division of the cochlear nucleus. Electron microscopic analysis confirms that mGluR1 alpha immunoreactivity is concentrated in the dendritic spines of cartwheel cells and in dendrites of the recently described unipolar brush cells. The cartwheel cells, which have many similarities to the Purkinje cells of the cerebellum, participate in a local neuronal circuit that modulates the output of the dorsal cochlear nucleus. Immunostained unipolar brush cells were observed in granule cell regions of the cochlear nucleus and the vestibulocerebellum. The presence of analogous cell types with similar patterns of immunolabeling in the cerebellum and in the dorsal cochlear nucleus suggests that a shared but as yet unknown mode of processing may occur in both structures. | | 8821458
 |