219465 Sigma-AldrichCelastrol, Celastrus scandens - CAS 34157-83-0 - Calbiochem
A cell-permeable dienone-phenolic triterpene compound that exhibits antioxidant and anti-inflammatory properties.
More>> A cell-permeable dienone-phenolic triterpene compound that exhibits antioxidant and anti-inflammatory properties. Less<<Synonyms: Tripterin, 3-Hydroxy-24-nor-2-oxo-1(10),3,5,7-friedelatetraen-29-oic Acid, Proteasome Inhibitor XIX
Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| CAS # | Empirical Formula |
|---|---|
| 34157-83-0 | C₂₉H₃₈O₄ |
Pricing & Availability
| Catalog Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 219465-10MG |
|
Plastic ampoule | 10 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 34157-83-0 |
| ATP Competitive | N |
| Form | Red crystals |
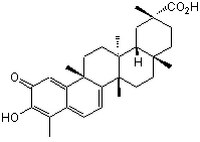
| Hill Formula | C₂₉H₃₈O₄ |
| Chemical formula | C₂₉H₃₈O₄ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | 20S proteasome |
| Primary Target IC<sub>50</sub> | 2.5 µM inhibiting chymotrypsin-like activity of 20S proteasome |
| Purity | ≥95% by TLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| 219465-10MG | 04055977218459 |
Documentation
Celastrol, Celastrus scandens - CAS 34157-83-0 - Calbiochem SDS
| Title |
|---|
Celastrol, Celastrus scandens - CAS 34157-83-0 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 219465 |
References
| Reference overview |
|---|
| Yang, H., et al. 2006. Cancer Res. 66, 4758. Westerheide, S.D., et al. 2004. J. Biol. Chem. 279, 56053. Jin, H.Z., et al. 2002. J. Nat. Prod. 65, 89. Allison, A.C., et al. 2001. Prog. Neuropsychopharmacol. Biol. Psychiatry 25, 1341. He, W., et al. 1998. Bioorg. Med. Chem. Lett. 8, 3659. Sassa, H., et al. 1990. Biochem. Biophys. Res. Commun. 172, 890. |