330175 Sigma-AldrichEchinomycin Streptomyces sp. - CAS 512-64-1 - Calbiochem
A cell-permeable Streptomyces-derived antibiotic that contains two bicyclic peptide-linked quinoxalines and acts as a sequence-specific DNA bisintercalator.
More>> A cell-permeable Streptomyces-derived antibiotic that contains two bicyclic peptide-linked quinoxalines and acts as a sequence-specific DNA bisintercalator. Less<<Synonyms: NSC-13502, Quinomycin A
Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| CAS # | Empirical Formula |
|---|---|
| 512-64-1 | C₅₁H₆₄N₁₂O₁₂S₂ |
| Product Information | |
|---|---|
| CAS number | 512-64-1 |
| Form | White to off-white to fawn solid |
| Hill Formula | C₅₁H₆₄N₁₂O₁₂S₂ |
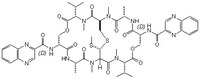
| Chemical formula | C₅₁H₆₄N₁₂O₁₂S₂ |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥98% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| 330175 | 0 |
Documentation
Echinomycin Streptomyces sp. - CAS 512-64-1 - Calbiochem SDS
| Title |
|---|
Echinomycin Streptomyces sp. - CAS 512-64-1 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 330175 |
References
| Reference overview |
|---|
| Park, J.Y., et al. 2008. Cell. Biol. Int. 32, 1207. Kong, D., et al. 2005. Cancer Res. 65, 9047. Chang, A.Y., et al. 1998. Cancer 82, 292. Wadler, S., et al. 1994. Cancer Chemother. Pharmacol. 34, 266. Muss, H.B., et al. 1993. Am. J. Clin. Oncol. 16, 492. Foster, B.J., et al. 1985. Invest. New Drugs 3, 403. Van Dyke, N.M., et al. 1984. Science 225, 1122. Ward, D.C., et al. 1965. Science 149, 1259. |