324693 Sigma-AldrichEmetine, Dihydrochloride - CAS 316-42-7 - Calbiochem
Principal alkaloid of ipecac, isolated from the ground roots of Uragoga ipecacuanha.
More>> Principal alkaloid of ipecac, isolated from the ground roots of Uragoga ipecacuanha. Less<<Synonyms: 6ʹ,7ʹ,10,11-Tetramethoxyemetan, 2HCl
Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| CAS # | Empirical Formula |
|---|---|
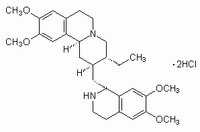
| 316-42-7 | C₂₉H₄₀N₂O₄ · 2HCl |
Pricing & Availability
| Catalog Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 324693-250MG |
|
Alu drum | 250 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 316-42-7 |
| ATP Competitive | N |
| Form | White to off-white solid |
| Hill Formula | C₂₉H₄₀N₂O₄ · 2HCl |
| Chemical formula | C₂₉H₄₀N₂O₄ · 2HCl |
| Hygroscopic | Hygroscopic |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Physicochemical Information | |
|---|---|
| Cell permeable | N |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | JY5250000 |
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| 324693-250MG | 04055977215885 |
Documentation
Emetine, Dihydrochloride - CAS 316-42-7 - Calbiochem SDS
| Title |
|---|
Emetine, Dihydrochloride - CAS 316-42-7 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 324693 |
References
| Reference overview |
|---|
| Kong, H.S., et al. 2010. Mol. Pharmacol. in press. Khan, M.A. 1995. Prog. Neurobiol. 46, 541. Kokuho, T., et al. 1995. Immunobiology 193, 42. Lee, Y.S., and Wurster, R.D. 1995. Cancer Lett. 93, 157. Burhans, W.C., et al. 1991. EMBO J. 10, 4351. Filley, E.A., and Rook, G.A. 1991. Infect. Immun. 59, 2567. Landis, R.C., et al. 1991. J. Immunol. 146, 128. Schweighoffer, T., et al. 1991. Histochemistry 96, 93. |