419820 Sigma-AldrichITK Inhibitor, BMS-509744 - CAS 439575-02-7 - Calbiochem
Synonyms: BMS509744; EMT Inhibitor, IL-2-Inducible T Cell Kinase Inhibitor, N-(5-(5-(4-Acetylpiperazine-1-carbonyl)-4-methoxy-2-methylphenylthio)thiazol-2-yl)-4-((3,3-dimethylbutan-2-ylamino)methyl)benzamide, N-(5-((3-((4-Acetylpiperazin-1-yl)carbonyl)-4-methyl-6-methoxyphenyl)thio)thiazol-2-yl)-4-(N-1,2-dimethylpropylaminomethyl)benzamide
Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| CAS # | Empirical Formula |
|---|---|
| 439575-02-7 | C₃₂H₄₁N₅O₄S₂ |
Pricing & Availability
| Catalog Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 419820-5MG |
|
Glass bottle | 5 mg |
|
— |
| Description | |
|---|---|
| Overview | A cell-permeable aminothioaryl-thiazolo compound that potently inhibits ITK kinase activity (IC50 = 19 nM; [ATP] = 1 µM) in an ATP-competitive manner by stabilizing ITK activation loop in a substrate-blocking, inactive conformation, inhibiting Fyn, IR, Lck, Btk only at much higher concentrations (IC50 ≥1.1 µM) and exhibiting little or no activity against 14 other kinases (IC50 ≥11 µM). Inhibits ITK-dependent cellular signaling (IC50 <300 nM against αCD3-stimulated PLCγ1 Tyr phosphorylation and Ca2+ mobilization in Jurkat cells) in vitro and alleviate OVA (Cat. No. 32467) challenge-induced airway leukocytes infiltration (ED50 = 25 mg/kg s.c.) in OVA-sensitized mice in vivo. |
| Catalogue Number | 419820 |
| Brand Family | Calbiochem® |
| Synonyms | BMS509744; EMT Inhibitor, IL-2-Inducible T Cell Kinase Inhibitor, N-(5-(5-(4-Acetylpiperazine-1-carbonyl)-4-methoxy-2-methylphenylthio)thiazol-2-yl)-4-((3,3-dimethylbutan-2-ylamino)methyl)benzamide, N-(5-((3-((4-Acetylpiperazin-1-yl)carbonyl)-4-methyl-6-methoxyphenyl)thio)thiazol-2-yl)-4-(N-1,2-dimethylpropylaminomethyl)benzamide |
| References | |
|---|---|
| References | Kutach, A.K., et al. 2010. Chem. Biol. Drug Des. 76, 154. Das, J., et al. 2006. Bioorg. Med. Chem. Lett. 16, 3706. Lin, T.A., et al. 2004. Biochemistry 43, 11056. |
| Product Information | |
|---|---|
| CAS number | 439575-02-7 |
| Form | Pale yellow powder |
| Hill Formula | C₃₂H₄₁N₅O₄S₂ |
| Chemical formula | C₃₂H₄₁N₅O₄S₂ |
| Reversible | Y |
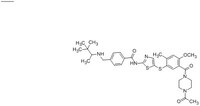
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | ITK |
| Primary Target IC<sub>50</sub> | 19 nM for ITK |
| Purity | ≥97% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| 419820-5MG | 04055977187830 |
Documentation
ITK Inhibitor, BMS-509744 - CAS 439575-02-7 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 419820 |
References
| Reference overview |
|---|
| Kutach, A.K., et al. 2010. Chem. Biol. Drug Des. 76, 154. Das, J., et al. 2006. Bioorg. Med. Chem. Lett. 16, 3706. Lin, T.A., et al. 2004. Biochemistry 43, 11056. |
| Data Sheet | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|