ECM642 Sigma-AldrichIn Vitro Vascular Permeability Assay (96-well)
This In Vitro Vascular Permeability Assay kit employs a 96-well plate, and provides an efficient system for evaluating the effects of chemicals & drug compounds on endothelial cell adsorption, transport & permeability.
More>> This In Vitro Vascular Permeability Assay kit employs a 96-well plate, and provides an efficient system for evaluating the effects of chemicals & drug compounds on endothelial cell adsorption, transport & permeability. Less<<Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| Detection Methods |
|---|
| Fluorescent |
| Description | |
|---|---|
| Catalogue Number | ECM642 |
| Brand Family | Chemicon® |
| Trade Name |
|
| Description | In Vitro Vascular Permeability Assay (96-well) |
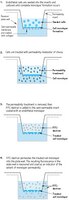
| Overview | NOW AVAILABLE! In Vitro Vascular Permeability Imaging Assay (17-10398) Read our application note in Nature Methods! http://www.nature.com/app_notes/nmeth/2012/121007/pdf/an8623.pdf (Click Here!) Introduction: A fundamental requirement for the physiological performance of organs is the formation of diffusion barriers that separate and maintain compartments of different structure. The endothelial cell lining of the internal vasculature defines a semi-permeable barrier between the blood and the interstitial spaces of the body. This barrier is composed of intercellular adherens, tight, and gap junction complexes, as well as desmosomes. Junction substructure components such as connexins, integrins, cadherins, catenins, occludins, desmoplakins, selectins, and platelet endothelial cell adhesion molecule-1 (PECAM-1) all act as interface regulators for paracellular permeability of ions, nutrients, therapeutic agents, and macromolecules. Endothelial cell adhesive characteristics provide strength and stability for neighboring cells and the cellular cytoskeleton by interacting with actin and myosin contractile filaments. Junctional molecules also influence cell signaling and trigger responses that are translated into cell morphology changes and physiological angiogenesis. A multitude of vasoactive cytokines, growth factors, and signal modulators react with endothelial cell substructural components to control permeability. Vascular endothelial growth factor (VEGF), interleukin-1 alpha and beta (IL-1α and IL-1β), tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ) have been shown to increase endothelial monolayer permeability. Thrombin stimulation of cytoskeletal signaling pathways has been shown to manipulate cell permeability. Lipopolysaccharide (LPS) induces junction barrier loss and cell detachment by activating protein tyrosine kinases (PTKs) and caspase cleavage reactions. In contrast, junctional adhesion molecule (JAM) decreases permeability by initiating cell adhesion and angiopoietin-1 (Ang-1) can protect endothelial barrier function through regulation of junctional complexes. Disruptions of the barrier integrity are manifested as microvascular hyperpermeability, which is associated with many systemic disease states. Pathological angiogenic disease states include heart disease, diabetes, cancer, stroke, hypertension, arthritis, and Alzheimer’s. Increases in tissue permeability may be caused by weak, hemorrhaging vessels that become oedematous, and intensifies with irregular fluid flow through the vessels. Expanding the knowledge of endothelial junction behavior and the agents that influence that behavior will lead to new therapies for controlling endothelial permeability. An essential ingredient of any in vitro permeability study is an intact, confluent cell monolayer. Endothelial cell monolayers cultured on semi-permeable membranes have been shown to form adherent and tight junctions. The Millipore In Vitro Vascular Permeability Assay kit provides an efficient system for evaluating the effects of chemicals and drug compounds on endothelial cell adsorption, transport, and permeability. Test Principle: The Millipore In Vitro Vascular Permeability Assay is performed in a 96-well receiver plate with 96 cell culture inserts connected in a single tray unit. The inserts contain 1 µm pores within a transparent polyethylene terephthalate (PET) membrane. Each insert has been pre-coated with an optimized concentration of type I rat-tail collagen. The high pore density membranes permit apical and basolateral access of cells to media and permeability molecules of interest. Within Millipore’s In Vitro Vascular Permeability Assay, endothelial cells are seeded onto the collagen-coated inserts. An endothelial monolayer forms in several days, which occludes the membrane pores. The cell monolayer is then treated with cytokines, growth factors, or other compounds of interest. After treatment, a high molecular weight FITC-Dextran is added on top of the cells, allowing the fluorescent molecules to pass through the endothelial cell monolayer at a rate proportional to the monolayer’s permeability. The extent of permeability can be determined by measuring the fluorescence of the receiver plate well solution. |
| Materials Required but Not Delivered | 1. Human umbilical vein endothelial cells (HUVEC) such as EndoGRO™ Human Umbilical Vein Endothelial Cells (Cat. No. SCCE001) or endothelial cell type of interest. 2. Endothelial cell Basal Medium. (Note: Phenol red should be avoided—it may interfere with fluorescence measurement and increase background fluorescence.) 3. Endothelial cell Growth Medium. 4. Vascular permeability factor (e.g., IL-1β, TNF-α, VEGF, Ang-1, etc.). 5. Cell detachment buffer (e.g., 0.05% trypsin). 6. Sterile phosphate-buffered saline (PBS) or Hank’s balanced salt solution (HBSS) for washing of cells. 7. Sterile cell culture hood. 8. Pipettors (e.g., multichannel, repeater), liquid aspirators, etc. for handling of cells and liquid reagents. 9. Sterile plasticware (cell culture flasks, centrifuge tubes, pipettes, pipette tips, reagent reservoirs, etc. for handling of cells and liquid reagents). 10. CO2 tissue culture incubator. 11. 70% ethanol for disinfection of plasticware, surfaces, etc. 12. Low speed centrifuge for cell harvesting. 13. Hemocytometer. 14. Trypan blue or equivalent viability stain. 15. Microscope (for phase contrast and brightfield imaging). 16. Fluorescence plate reader with filters for 485 nm and 535 nm excitation and emission, respectively (appropriate for FITC/fluorescein signal). |
| References |
|---|
| Product Information | |
|---|---|
| Components |
|
| Detection method | Fluorescent |
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Species Reactivity |
|
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Storage and Shipping Information | |
|---|---|
| Storage Conditions | Store kit materials at 2-8°C; use within 4 months from date of receipt. Do not freeze. |
| Packaging Information | |
|---|---|
| Material Size | 96 assays |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| ECM642 | 04053252413858 |
Documentation
In Vitro Vascular Permeability Assay (96-well) SDS
| Title |
|---|
Technical Info
| Title |
|---|
| 96 well Template 2 Up |
| 96 well Template Single |
User Guides
| Title |
|---|
| In Vitro Vascular Permeability Assay (96-Well) |