530657 Sigma-AldrichMyosin ATPase Activator, EMD57003 - CAS 147527-31-9 - Calbiochem
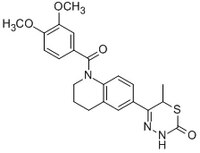
A cell-permeable, isomer of the racemate EMD-53998. Binds to the allosteric pocket of myosin motor domain and stimulates actomyosin ATPase activity (AC₅₀ = 7.0 µM for β-cardiac myosin)
More>> A cell-permeable, isomer of the racemate EMD-53998. Binds to the allosteric pocket of myosin motor domain and stimulates actomyosin ATPase activity (AC₅₀ = 7.0 µM for β-cardiac myosin) Less<<Synonyms: (+)-5-(1-(3,4-Dimethoxybenzoyl)-1,2,3,4-tetrahydroquinolin-6-yl)-6-methyl-3,6-dihydro-2H-1,3,4-thiadiazin-2-one, (+)-6-(3,6-Dihydro-6-methyl-2-oxo-2H-1,3,4-thiadiazin-5-yl)-1-(3,4-dimethoxybenzoyl)-1,2,3,4-tetrahydroquinoline, EMD-57033, Myosin Motor Activator
Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| CAS # | Empirical Formula |
|---|---|
| 147527-31-9 | C₂₂H₂₃N₃O₄S |
| Product Information | |
|---|---|
| CAS number | 147527-31-9 |
| Form | Off-white solid |
| Hill Formula | C₂₂H₂₃N₃O₄S |
| Chemical formula | C₂₂H₂₃N₃O₄S |
| Reversible | Y |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | myosin |
| Purity | ≥97% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| 530657 | 0 |
Documentation
Myosin ATPase Activator, EMD57003 - CAS 147527-31-9 - Calbiochem SDS
| Title |
|---|
Myosin ATPase Activator, EMD57003 - CAS 147527-31-9 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 530657 |
References
| Reference overview |
|---|
| Radke, M.B., et al. 2014. ELife 3, e01603. Senzaki, H., et al., 2000. Circulation 101, 1040. Gambassi, G., et al. 1993. Am. J. Physiol. 264, H728. Solaro, R.J., et al.1993. Circ. Res.73, 981. |