521275 Sigma-AldrichPDK1/Akt/Flt Dual Pathway Inhibitor - CAS 331253-86-2 - Calbiochem
The PDK1/Akt/Flt Dual Pathway Inhibitor, also referenced under CAS 331253-86-2, controls the biological activity of PDK1/Akt/Flt. This small molecule/inhibitor is primarily used for Phosphorylation & Dephosphorylation applications.
More>> The PDK1/Akt/Flt Dual Pathway Inhibitor, also referenced under CAS 331253-86-2, controls the biological activity of PDK1/Akt/Flt. This small molecule/inhibitor is primarily used for Phosphorylation & Dephosphorylation applications. Less<<Synonyms: 6H-Indeno[1,2-e]tetrazolo[1,5-b][1,2,4]triazin-6-one & 10H-Indeno[2,1-e]tetrazolo[1,5-b][1,2,4]triazin-10-one, Akt Inhibitor XXI, PDK1 Inhibitor I, KP372-1
Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| CAS # | Empirical Formula |
|---|---|
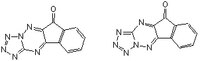
| 331253-86-2 | C₁₀H₄N₆O |
Pricing & Availability
| Catalog Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 521275-5MG |
|
Plastic ampoule | 5 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 331253-86-2 |
| ATP Competitive | N |
| Form | Yellow solid |
| Hill Formula | C₁₀H₄N₆O |
| Chemical formula | C₁₀H₄N₆O |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| 521275-5MG | 04055977271447 |
Documentation
PDK1/Akt/Flt Dual Pathway Inhibitor - CAS 331253-86-2 - Calbiochem SDS
| Title |
|---|
PDK1/Akt/Flt Dual Pathway Inhibitor - CAS 331253-86-2 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 521275 |
References
| Reference overview |
|---|
| Zeng, Z., et al. 2006. Cancer Res. 66, 3737. Koul, D., et al. 2006. Mol. Cancer Ther. 5, 637. Mandal. M., et al. 2006. Oral Oncol. 42, 430. Mandal. M., et al. 2005. Br. J. Cancer 92, 1899. |
Brochure
| Title |
|---|
| Biologics 33.2 |