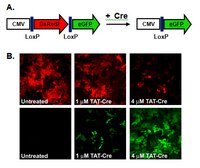
Excision of viral reprogramming cassettes by Cre protein transduction enables rapid, robust and efficient derivation of transgene-free human induced pluripotent stem cells.
Kadari, Asifiqbal, et al.
Stem Cell Res Ther, 5: 47 (2014)
2014
Show Abstract
Integrating viruses represent robust tools for cellular reprogramming, however, the presence of viral transgenes in induced pluripotent stem cells (iPSCs) is deleterious as it holds the risk of insertional mutagenesis leading to malignant transformation. Here, we combine the robustness of lentiviral reprogramming with the efficacy of Cre recombinase protein transduction to derive iPSCs devoid of transgenes. By genome-wide analysis and targeted differentiation towards the cardiomyocyte lineage, we show that transgene-free iPSCs are superior to iPSCs before Cre transduction. Our study provides a simple, rapid and robust protocol for the generation of clinical-grade iPSCs suitable for disease modeling, tissue engineering and cell replacement therapies. | 24713299
 |
Stage-specific conditional mutagenesis in mouse embryonic stem cell-derived neural cells and postmitotic neurons by direct delivery of biologically active Cre recombinase.
Haupt, Simone, et al.
Stem Cells, 25: 181-8 (2007)
2007
Show Abstract
Conditional mutagenesis using Cre/loxP recombination is a powerful tool to investigate genes involved in neural development and function. However, the efficient delivery of biologically active Cre recombinase to neural cells, particularly to postmitotic neurons, represents a limiting factor. In this study, we devised a protocol enabling highly efficient conditional mutagenesis in ESC-derived neural progeny. Using a stepwise in vitro differentiation paradigm, we demonstrate that recombinant cell-permeable Cre protein can be used to efficiently induce recombination at defined stages of neural differentiation. Recombination rates of more than 90% were achieved in multipotent pan-neural and glial precursors derived from the Z/EG reporter mouse ESC line, in which Cre recombination activates enhanced green fluorescent proteinexpression. Recombined precursor cells displayed a normal phenotype and were able to differentiate into neurons and/or glial cells, indicating that Cre treatment has no overt side effects on proliferation and neural differentiation. Our data further demonstrate that recombination via Cre protein transduction is not restricted to dividing cells but can even be applied to postmitotic neurons. The ability to conduct Cre/loxP recombination at defined stages of stem cell differentiation in an expression-independent manner provides new prospects for studying the role of individual genes under stringent temporal control. | 16960133
 |
Stem cell engineering using transducible Cre recombinase.
Nolden, Lars, et al.
Methods Mol. Med., 140: 17-32 (2007)
2007
Show Abstract
Embryonic stem (ES) cells have become a major focus of scientific interest both as a potential donor source for regenerative medicine and as a model system for tissue development and pathobiology. Tight and efficient methods for genetic engineering are required to exploit ES cells as disease models and to generate specific somatic phenotypes by lineage selection or instruction. In 1990s, the application of site-specific recombinases (SSRs) such as Cre has revolutionized mammalian genetics by providing a reliable and efficient means to delete, insert, invert, or exchange chromosomal DNA in a conditional manner. Despite these significant advances, the available technology still suffers from limitations, including unwanted side effects elicited by the random integration of Cre expression vectors and leak activity of inducible or presumptive cell type-specific Cre expression systems. These challenges can be met by combining the Cre/loxP recombination system with direct intracellular delivery of Cre by protein transduction, thus enabling rapid and highly efficient conditional mutagenesis in ES cells and ES cell-derived somatic progeny. Modified recombinant variants of Cre protein induce recombination in virtually 100% of human ES (hES) and mouse ES (mES) cells. Here, we present methods for generating purified transducible Cre protein from Escherichia coli and its transduction into ES cells and their neural progeny. | 18085201
 |
Site-specific recombination in human embryonic stem cells induced by cell-permeant Cre recombinase.
Nolden, Lars, et al.
Nat. Methods, 3: 461-7 (2006)
2006
Show Abstract
The biomedical application of human embryonic stem (hES) cells will increasingly depend on the availability of technologies for highly controlled genetic modification. In mouse genetics, conditional mutagenesis using site-specific recombinases has become an invaluable tool for gain- and loss-of-function studies. Here we report highly efficient Cre-mediated recombination of a chromosomally integrated loxP-modified allele in hES cells and hES cell-derived neural precursors by protein transduction. Recombinant modified Cre recombinase protein translocates into the cytoplasm and nucleus of hES cells and subsequently induces recombination in virtually 100% of the cells. Cre-transduced hES cells maintain the expression of pluripotency markers as well as the capability of differentiating into derivatives of all three germ layers in vitro and in vivo. We expect this technology to provide an important technical basis for analyzing complex genetic networks underlying human development as well as generating highly purified, transplantable hES cell-derived cells for regenerative medicine. | 16721380
 |