Pattern of Cell Proliferation During Budding in the Colonial Ascidian Diplosoma listerianum.
Sköld HN, Stach T, Bishop JD, Herbst E, Thorndyke MC
The Biological bulletin
221
126-36.
2011
Show Abstract
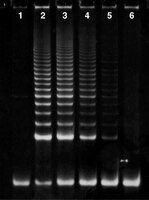
Many invertebrates reproduce asexually by budding, but morphogenesis and the role of cell proliferation in this diverse and nonconserved regeneration-like process are generally poorly understood and particularly little investigated in didemnid ascidians. We here analyzed cell proliferation patterns and telomerase activity during budding in the colonial didemnid ascidian Diplosoma listerianum, with special focus on the thoracic bud where a new brain develops de novo. To help define developmental stages of the thoracic bud, the distribution of acetylated tubulin was also examined. We found extensive cell proliferation in both the thoracic and abdominal buds of D. listerianum as well as higher telomerase activity in bud tissue compared to adult tissues. In the parent adult, proliferation was found in various tissues, but was especially intense in the adult esophagus and epicardial structures that protrude into the proliferating and developing buds, confirming these tissues as the primary source of the cells that form the buds. The neural complex in the thoracic bud forms from a hollow tube that appears to separate into the neural gland and the cerebral ganglion. Whereas most of the bud undergoes proliferation, including the hollow tube and the neural gland, the cerebral ganglion shows little or no proliferation. Pulse-chase labeling experiments indicate that the ganglion, as well as the myocardium, in adult zooids are instead composed of postmitotic cells. | | | 21876115
 |
Detection of telomerase activity in high concentration of cell lysates using primer-modified gold nanoparticles.
Yi Xiao,Karen Y Dane,Takanori Uzawa,Andrew Csordas,Jiangrong Qian,H Tom Soh,Patrick S Daugherty,Eric T Lagally,Alan J Heeger,Kevin W Plaxco
Journal of the American Chemical Society
132
2010
Show Abstract
Although the telomeric repeat amplification protocol (TRAP) has served as a powerful assay for detecting telomerase activity, its use has been significantly limited when performed directly in complex, interferant-laced samples. In this work, we report a modification of the TRAP assay that allows the detection of high-fidelity amplification of telomerase products directly from concentrated cell lysates. Briefly, we covalently attached 12 nm gold nanoparticles (AuNPs) to the telomere strand (TS) primer, which is used as a substrate for telomerase elongation. These TS-modified AuNPs significantly reduce polymerase chain reaction (PCR) artifacts (such as primer dimers) and improve the yield of amplified telomerase products relative to the traditional TRAP assay when amplification is performed in concentrated cell lysates. Specifically, because the TS-modified AuNPs eliminate most of the primer-dimer artifacts normally visible at the same position as the shortest amplified telomerase PCR product apparent on agarose gels, the AuNP-modified TRAP assay exhibits excellent sensitivity. Consequently, we observed a 10-fold increase in sensitivity for cancer cells diluted 1000-fold with somatic cells. It thus appears that the use of AuNP-modified primers significantly improves the sensitivity and specificity of the traditional TRAP assay and may be an effective method by which PCR can be performed directly in concentrated cell lysates. | | | 20932008
 |
3D nuclear organization of telomeres in the Hodgkin cell lines U-HO1 and U-HO1-PTPN1: PTPN1 expression prevents the formation of very short telomeres including t-stumps.
Hans Knecht,Silke Brüderlein,Silke Wegener,Daniel Lichtensztejn,Zelda Lichtensztejn,Bruno Lemieux,Peter Möller,Sabine Mai
BMC cell biology
11
2010
Show Abstract
In cancer cells the three-dimensional (3D) telomere organization of interphase nuclei into a telomeric disk is heavily distorted and aggregates are found. In Hodgkin's lymphoma quantitative FISH (3D Q-FISH) reveals a major impact of nuclear telomere dynamics during the transition form mononuclear Hodgkin (H) to diagnostic multinuclear Reed-Sternberg (RS) cells. In vitro and in vivo formation of RS-cells is associated with the increase of very short telomeres including t-stumps, telomere loss, telomeric aggregate formation and the generation of ghost nuclei. Full Text Article | | | 21144060
 |
Establishment and characterization of a human retinal pericyte line: a novel tool for the study of diabetic retinopathy.
Berrone, Elena, et al.
Int. J. Mol. Med., 23: 373-8 (2009)
2009
Show Abstract
Loss of pericytes from retinal microvessels is one of the key events in the natural history of diabetic retinopathy. Cultured human retinal pericytes would constitute an extremely useful tool for the study of the early events in the pathogenesis of this complication, but, due to legal and ethical issues, pericytes of animal origin have been mostly used so far for in vitro assays. We aimed at establishing an immortalized human retinal pericyte (HRP) line, as a species-specific model to investigate the pericyte-related aspects of diabetic retinopathy. Primary human retinal pericytes (WT-HRP) were immortalized through electroporation with a plasmid vector containing the Bmi-1 oncogene that induces telomerase activity, resulting in the establishment of a permanent pericyte line (Bmi-HRP), which showed telomerase activity and facilitated propagation. The immortalized cells were characterized for typical pericyte morphology and marker expression. Immunofluorescence studies demonstrated that Bmi-HRP maintain the same morphology and express the typical markers of wild-type pericytes. The response of the cell line to high glucose damaging stimulus was also evaluated, as senescence-associated beta-galactosidase activity and cell proliferation and a clear negative effect of high glucose on Bmi-HRP proliferation and senescence, in line with the characteristic response of wild-type cells, was observed. The combination of infinite proliferation capability and stable differentiation potential makes our Bmi-HRP line a promising candidate model to study pathogenic mechanisms and therapeutic applications in diabetic retinopathy. | Immunofluorescence | Human | 19212656
 |
Hypomethylation of subtelomeric regions in ICF syndrome is associated with abnormally short telomeres and enhanced transcription from telomeric regions.
Shiran Yehezkel, Yardena Segev, Evani Viegas-Péquignot, Karl Skorecki, Sara Selig
Human molecular genetics
17
2776-89
2008
Show Abstract
Telomeres and adjacent subtelomeric regions are packaged as heterochromatin in many organisms. The heterochromatic features include DNA methylation, histones H3-Lys9 (Lysine 9) and H4-Lys20 (Lysine 20) methylation and heterochromatin protein1 alpha binding. Subtelomeric DNA is hypomethylated in human sperm and ova, and these regions are subjected to de novo methylation during development. In mice this activity is carried out by DNA methyltransferase 3b (Dnmt3b). Mutations in DNMT3B in humans lead to the autosomal-recessive ICF (immunodeficiency, centromeric region instability, facial anomalies) syndrome. Here we show that, in addition to several satellite and non-satellite repeats, the subtelomeric regions in lymphoblastoid and fibroblast cells of ICF patients are also hypomethylated to similar levels as in sperm. Furthermore, the telomeres are abnormally short in both the telomerase-positive and -negative cells, and many chromosome ends lack detectable telomere fluorescence in situ hybridization signals from either one or both sister-chromatids. In contrast to Dnmt3a/b(-/-) mouse embryonic stem cells, increased telomere sister-chromatid exchange was not observed in ICF cells. Hypomethylation of subtelomeric regions was associated in the ICF cells with advanced telomere replication timing and elevated levels of transcripts emanating from telomeric regions, known as TERRA (telomeric-repeat-containing RNA) or TelRNA. The current findings provide a mechanistic explanation for the abnormal telomeric phenotype observed in ICF syndrome and highlights the link between TERRA/TelRNA and structural telomeric integrity. | | | 18558631
 |
Evaluation of telomere length maintenance mechanisms in adrenocortical carcinoma.
Tobias Else,Thomas J Giordano,Gary D Hammer
The Journal of clinical endocrinology and metabolism
93
2008
Show Abstract
Adrenocortical cancer (ACC) is a rare disease with an often fatal outcome. The clinical and pathological diagnosis of a malignant vs. benign adrenocortical tumor is sometimes challenging. Telomere maintenance mechanisms (TMMs) are critical for the persistence of the malignant phenotype, but little is known about these mechanisms or their diagnostic value in adrenocortical lesions. | | Human | 18198226
 |
Knockdown of p53 combined with expression of the catalytic subunit of telomerase is sufficient to immortalize primary human ovarian surface epithelial cells.
Gong Yang, Daniel G Rosen, Imelda Mercado-Uribe, Justin A Colacino, Gordon B Mills, Robert C Bast, Chenyi Zhou, Jinsong Liu
Carcinogenesis
28
174-82
2007
Show Abstract
Ovarian cancer is developed from a single layer of thin epithelial cells covering the surface of ovary, named human ovarian surface epithelial cells. Like all primary human cells, human ovarian surface epithelial cells have a finite life span and will go into senescence and eventually die when cultured in vitro. Immortalized human ovarian surface epithelial cells will provide an important model system with which to study ovarian cancer initiation and progression. Here, we show that silencing p53 expression with retrovirus-mediated small interfering RNA can delay the senescence and extend cell passage number, but is not sufficient to immortalize normal ovarian surface epithelial cells. Introduction of the catalytic subunit of telomerase is similarly insufficient to achieve immortalization. However, concurrent disruption of p53 expression with small interfering RNA retroviral constructs and ectopic expression of the catalytic subunit of telomerase was sufficient to induce cellular immortalization in 3 of 3 human ovarian surface epithelial cell cultures tested. The immortalization is associated with increased telomerase activity and telomere length, and attenuated response of cell-cycle regulatory proteins to irradiation. The resultant immortal cells continued to express the same specific cytokeratins 8 and 18 as parental cells did, indicating that the epithelial characters are still maintained in the immortal cells. In addition, the immortalized cells are non-tumorigenic and nearly diploid, which is in constrast with one immortalized by SV40 T/t antigens and hTERT. As both p53 pathway dysfunction and activation of telomerase are commonly present in human ovarian cancer, these immortal cells provide an authetic cell model system for the study of the human ovarian cancer initiation, progression, differentiation and chemoprevention. | | | 16829690
 |
Telomere dynamics in macaques and humans.
Jeffrey P Gardner, Masayuki Kimura, Weihang Chai, Jameel F Durrani, Levon Tchakmakjian, Xiaojian Cao, Xiaobin Lu, Guanghui Li, Athanasios P Peppas, Joan Skurnick, Woodring E Wright, Jerry W Shay, Abraham Aviv
The journals of gerontology. Series A, Biological sciences and medical sciences
62
367-74
2007
Show Abstract
In humans, telomere length in proliferating tissues shortens with age--a process accelerated with age-related diseases. Thus, telomere length and attrition with age in the nonhuman primate may serve as a useful paradigm for understanding telomere biology in humans. We examined telomere parameters in tissues of young and old Macaca fascicularis and compared them with several tissues from humans. Macaque telomeres were variable in length and exhibited partial synchrony (equivalence) within animals. They were longer than humans, partially because of longer subtelomeric segments. As skeletal muscle telomere length was unchanged with age, we used it as an internal reference to offset interanimal variation in telomere length. We identified age-dependent telomere attrition in lung, pancreas, skin, and thyroid. Similar to humans, telomerase activity was detected in spleen, thymus, digestive tract, and gonads. We conclude that factors that modify telomere attrition and aging in humans may also operate in the macaque. | | | 17452729
 |
Cationic corrole derivatives: a new family of G-quadruplex inducing and stabilizing ligands.
Boqiao Fu,Jing Huang,Lige Ren,Xiaocheng Weng,Yangyang Zhou,Yuhao Du,Xiaojun Wu,Xiang Zhou,Guangfu Yang
Chemical communications (Cambridge, England)
2007
Show Abstract
Water-soluble cationic corrole derivatives were designed and synthesized, and the first observation of their interactions with the telomeric G-quadruplex was made. | | | 17668095
 |
Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals.
Sunday O Akintoye, Thanh Lam, Songtao Shi, Jaime Brahim, Michael T Collins, Pamela G Robey
Bone
38
758-68
2006
Show Abstract
Autologous grafts from axial and appendicular bones commonly used to repair orofacial bone defects often result in unfavorable outcome. This clinical observation, along with the fact that many bone abnormalities are limited to craniofacial bones, suggests that there are significant differences in bone metabolism in orofacial, axial and appendicular bones. It is plausible that these differences are dictated by site-specificity of embryological progenitor cells and osteogenic properties of resident multipotent human bone marrow stromal cells (hBMSCs). This study investigated skeletal site-specific phenotypic and functional differences between orofacial (maxilla and mandible) and axial (iliac crest) hBMSCs in vitro and in vivo. Primary cultures of maxilla, mandible and iliac crest hBMSCs were established with and without osteogenic inducers. Site-specific characterization included colony forming efficiency, cell proliferation, life span before senescence, relative presence of surface markers, adipogenesis, osteogenesis and transplantation in immunocompromised mice to compare bone regenerative capacity. Compared with iliac crest cells, orofacial hBMSCs (OF-MSCs) proliferated more rapidly with delayed senescence, expressed higher levels of alkaline phosphatase and demonstrated more calcium accumulation in vitro. Cells isolated from the three skeletal sites were variably positive for STRO 1, a marker of hBMSCs. OF-MSCs formed more bone in vivo, while iliac crest hBMSCs formed more compacted bone that included hematopoietic tissue and were more responsive in vitro and in vivo to osteogenic and adipogenic inductions. These data demonstrate that hBMSCs from the same individuals differ in vitro and in vivo in a skeletal site-specific fashion and identified orofacial marrow stromal cells as unique cell populations. Further understanding of site-specific properties of hBMSCs and their impact on site-specific bone diseases and regeneration are needed. | | | 16403496
 |