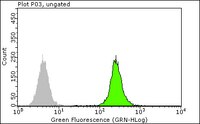
SERBP1 affects homologous recombination-mediated DNA repair by regulation of CtIP translation during S phase.
Ahn, JW; Kim, S; Na, W; Baek, SJ; Kim, JH; Min, K; Yeom, J; Kwak, H; Jeong, S; Lee, C; Kim, SY; Choi, CY
Nucleic acids research
43
6321-33
2015
Show Abstract
DNA double-strand breaks (DSBs) are the most severe type of DNA damage and are primarily repaired by non-homologous end joining (NHEJ) and homologous recombination (HR) in the G1 and S/G2 phase, respectively. Although CtBP-interacting protein (CtIP) is crucial in DNA end resection during HR following DSBs, little is known about how CtIP levels increase in an S phase-specific manner. Here, we show that Serpine mRNA binding protein 1 (SERBP1) regulates CtIP expression at the translational level in S phase. In response to camptothecin-mediated DNA DSBs, CHK1 and RPA2 phosphorylation, which are hallmarks of HR activation, was abrogated in SERBP1-depleted cells. We identified CtIP mRNA as a binding target of SERBP1 using RNA immunoprecipitation-coupled RNA sequencing, and confirmed SERBP1 binding to CtIP mRNA in S phase. SERBP1 depletion resulted in reduction of polysome-associated CtIP mRNA and concomitant loss of CtIP expression in S phase. These effects were reversed by reconstituting cells with wild-type SERBP1, but not by SERBP1 ΔRGG, an RNA binding defective mutant, suggesting regulation of CtIP translation by SERBP1 association with CtIP mRNA. These results indicate that SERBP1 affects HR-mediated DNA repair in response to DNA DSBs by regulation of CtIP translation in S phase. | 26068472
 |
TIMELESS Forms a Complex with PARP1 Distinct from Its Complex with TIPIN and Plays a Role in the DNA Damage Response.
Young, LM; Marzio, A; Perez-Duran, P; Reid, DA; Meredith, DN; Roberti, D; Star, A; Rothenberg, E; Ueberheide, B; Pagano, M
Cell reports
13
451-9
2015
Show Abstract
PARP1 is the main sensor of single- and double-strand breaks in DNA and, in building chains of poly(ADP-ribose), promotes the recruitment of many downstream signaling and effector proteins involved in the DNA damage response (DDR). We show a robust physical interaction between PARP1 and the replication fork protein TIMELESS, distinct from the known TIMELESS-TIPIN complex, which activates the intra-S phase checkpoint. TIMELESS recruitment to laser-induced sites of DNA damage is dependent on its binding to PARP1, but not PARP1 activity. We also find that the PARP1-TIMELESS complex contains a number of established PARP1 substrates, and TIMELESS mutants unable to bind PARP1 are impaired in their ability to bind PARP1 substrates. Further, PARP1 binding to certain substrates and their recruitment to DNA damage lesions is impaired by TIMELESS knockdown, and TIMELESS silencing significantly impairs DNA double-strand break repair. We hypothesize that TIMELESS cooperates in the PARP1-mediated DDR. | 26456830
 |