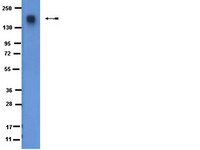
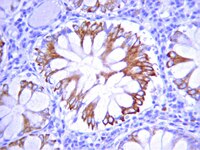
Cross-talk between epidermal growth factor receptor and c-Met signal pathways in transformed cells.
Jo, M, et al.
J. Biol. Chem., 275: 8806-11 (2000)
2000
Show Abstract
In rat liver epithelial cells constitutively expressing transforming growth factor alpha (TGFalpha), c-Met is constitutively phosphorylated in the absence of its ligand, hepatocyte growth factor. We proposed that TGFalpha and the autocrine activation of its receptor, epidermal growth factor receptor (EGFR), leads to phosphorylation and activation of c-Met. We found that there is constitutive c-Met phosphorylation in human hepatoma cell lines and the human epidermoid carcinoma cell line, A431 which express TGFalpha, but not in normal human hepatocytes. Constitutive c-Met phosphorylation in A431, HepG2, AKN-1, and HuH6 cells was inhibited by neutralizing antibodies against TGFalpha and/or EGFR. Exposure to exogenous TGFalpha or EGF increased the phosphorylation of c-Met in the human epidermoid carcinoma cell line, A431. The increase of c-Met phosphorylation by TGFalpha in A431 cells was inhibited by neutralizing antibodies against TGFalpha and/or EGFR and by the EGFR-specific inhibitor tyrphostin AG1478. These results indicate that constitutive c-Met phosphorylation, and the increase of c-Met phosphorylation by TGFalpha or EGF, in tumor cell lines is the result of the activation via EGFR. We found that c-Met in tumor cells co-immunoprecipitates with EGFR regardless of the existence of their ligands in tumor cells, but not in normal human hepatocytes. We conclude that c-Met associates with EGFR in tumor cells, and this association facilitates the phosphorylation of c-Met in the absence of hepatocyte growth factor. This cross-talk between c-Met and EGFR may have significant implications for altered growth control in tumorigenesis. | | 10722725
 |
Integration of growth factor, extracellular matrix, and retinoid signals during bronchial epithelial cell differentiation.
Moghal, N and Neel, B G
Mol. Cell. Biol., 18: 6666-78 (1998)
1998
Show Abstract
Epithelial cell differentiation is regulated by specific combinations of growth factors, hormones, and extracellular matrix (ECM). How these divergent signals are integrated is largely unknown. We used primary cultures of normal human bronchial epithelial cells (NHBEs) to investigate mechanisms of signal integration. In defined, serum-free media, NHBEs undergo mucosecretory differentiation only when grown in the presence of retinoids and on the appropriate substratum (collagen gels). We identified the retinoic acid receptor beta (RARbeta) gene as an early marker of NHBE differentiation. In contrast to immortalized cell lines, in NHBEs strong retinoid-induced RARbeta transcription occurs only when cells are grown on collagen gels, and it requires new protein synthesis and a cis-acting element that maps outside the known RARbeta promoter elements. NHBEs grown on collagen gels exhibit reduced epidermal growth factor (EGF)-induced Raf, MEK, and mitogen-activated protein kinase (MAPK) activity. This correlates with a specific inability to achieve high levels of p66(SHC) tyrosyl phosphorylation and association of p66(SHC) with GRB2, despite high levels of EGF receptor (EGFR) autophosphorylation. Notably, inhibition of EGFR or MEK/MAPK activation replaces the ECM requirement for RARbeta induction. Our results strongly suggest that a key mechanism by which specific ECMs facilitate retinoid-induced mucosecretory differentiation of NHBEs is by restricting the level of EGFR-dependent MEK/MAPK activation evoked by autocrine and/or paracrine EGFR ligands. | Neutralization Assay | 9774681
 |
Response diversity and the timing of progenitor cell maturation are regulated by developmental changes in EGFR expression in the cortex.
Burrows, R C, et al.
Neuron, 19: 251-67 (1997)
1997
Show Abstract
Early cortical progenitor cells of the ventricular zone (VZ) differ from later progenitor cells of the subventricular zone (SVZ) in cell-type generation and their level of epidermal growth factor receptors (EGFRs). To determine whether differences in their behavior are causally related to EGFR number/density, we introduced extra EGFRs into VZ cells with a retrovirus in vivo and in vitro. This results in premature expression of traits characteristic of late SVZ progenitor cells, including migration patterns, differentiation into astrocytes, and proliferation of multipotential cells to form spheres. The choice between proliferation and differentiation depends on ligand concentration and progenitor cell age and may reflect different thresholds of stimulation. The level of EGFRs expressed by progenitor cells in the cortex may therefore contribute to the timing of their maturation and choice of response to pleiotropic environmental signals. | Immunocytochemistry | 9292717
 |