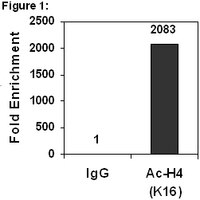
Histone deacetylation of NIS promoter underlies BRAF V600E-promoted NIS silencing in thyroid cancer.
Zhang, Z; Liu, D; Murugan, AK; Liu, Z; Xing, M
Endocrine-related cancer
21
161-73
2014
Show Abstract
The BRAF V600E mutation causes impaired expression of sodium iodide symporter (NIS) and radioiodine refractoriness of thyroid cancer, but the underlying mechanism remains undefined. In this study, we hypothesized that histone deacetylation at the NIS (SLC5A5) promoter was the mechanism. Using the chromatin immunoprecipitation approach, we examined histone acetylation status on the lysine residues H3K9/14, H3K18, total H4, and H4K16 at the NIS promoter under the influence of BRAF V600E. We found that expression of stably or transiently transfected BRAF V600E inhibited NIS expression while the deacetylase inhibitor SAHA stimulated NIS expression in PCCL3 rat thyroid cells. Although BRAF V600E enhanced global histone acetylation, it caused histone deacetylation at the NIS promoter while SAHA caused acetylation in the cells. In human thyroid cancer BCPAP cells harboring homozygous BRAF V600E mutation, BRAF V600E inhibitor, PLX4032, and MEK inhibitor, AZD6244, increased histone acetylation of the NIS promoter, suggesting that BRAF V600E normally maintained histone in a deacetylated state at the NIS promoter. The regions most commonly affected with deacetylation by BRAF V600E were the transcriptionally active areas upstream of the translation start that contained important transcription factor binding sites, including nucleotides -297/-107 in the rat NIS promoter and -692/-370 in the human NIS promoter. Our findings not only reveal an epigenetic mechanism for BRAF V600E-promoted NIS silencing involving histone deacetylation at critical regulatory regions of the NIS promoter but also provide further support for our previously proposed combination therapy targeting major signaling pathways and histone deacetylase to restore thyroid gene expression for radioiodine treatment of thyroid cancer. | 24243688
 |
Epigenetic suppression of mouse Per2 expression in the suprachiasmatic nucleus by the inhalational anesthetic, sevoflurane.
Mori, K; Iijima, N; Higo, S; Aikawa, S; Matsuo, I; Takumi, K; Sakamoto, A; Ozawa, H
PLoS One
9
e87319
2014
Show Abstract
We previously reported that sevoflurane anesthesia reversibly suppresses the expression of the clock gene, Period2 (Per2), in the mouse suprachiasmatic nucleus (SCN). However, the molecular mechanisms underlying this suppression remain unclear. In this study, we examined the possibility that sevoflurane suppresses Per2 expression via epigenetic modification of the Per2 promoter.Mice were anesthetized with a gas mixture of 2.5% sevoflurane/40% oxygen at a 6 L/min flow for 1 or 4 h. After termination, brains were removed and samples of SCN tissue were derived from frozen brain sections. Chromatin immunoprecipitation (ChIP) assays using anti-acetylated-histone antibodies were performed to investigate the effects of sevoflurane on histone acetylation of the Per2 promoter. Interaction between the E'-box (a cis-element in the Per2 promoter) and CLOCK (the Clock gene product) was also assessed by a ChIP assay using an anti-CLOCK antibody. The SCN concentration of nicotinamide adenine dinucleotide (NAD(+)), a CLOCK regulator, was assessed by liquid chromatography-mass spectrometry.Acetylation of histone H4 in the proximal region of the Per2 promoter was significantly reduced by sevoflurane. This change in the epigenetic profile of the Per2 gene was observed prior to suppression of Per2 expression. Simultaneously, a reduction in the CLOCK-E'-box interaction in the Per2 promoter was observed. Sevoflurane treatment did not affect the concentration of NAD(+) in the SCN.Independent of NAD(+) concentration in the SCN, sevoflurane decreases CLOCK binding to the Per2 promoter E'-box motif, reducing histone acetylation and leading to suppression of Per2 expression. | 24498074
 |
The histone acetyltransferase MOF activates hypothalamic polysialylation to prevent diet-induced obesity in mice.
Brenachot, X; Rigault, C; Nédélec, E; Laderrière, A; Khanam, T; Gouazé, A; Chaudy, S; Lemoine, A; Datiche, F; Gascuel, J; Pénicaud, L; Benani, A
Molecular metabolism
3
619-29
2014
Show Abstract
Overfeeding causes rapid synaptic remodeling in hypothalamus feeding circuits. Polysialylation of cell surface molecules is a key step in this neuronal rewiring and allows normalization of food intake. Here we examined the role of hypothalamic polysialylation in the long-term maintenance of body weight, and deciphered the molecular sequence underlying its nutritional regulation. We found that upon high fat diet (HFD), reduced hypothalamic polysialylation exacerbated the diet-induced obese phenotype in mice. Upon HFD, the histone acetyltransferase MOF was rapidly recruited on the St8sia4 polysialyltransferase-encoding gene. Mof silencing in the mediobasal hypothalamus of adult mice prevented activation of the St8sia4 gene transcription, reduced polysialylation, altered the acute homeostatic feeding response to HFD and increased the body weight gain. These findings indicate that impaired hypothalamic polysialylation contribute to the development of obesity, and establish a role for MOF in the brain control of energy balance. | 25161885
 |
Functional complementation of sir2Δ yeast mutation by the human orthologous gene SIRT1.
Gaglio, D; D'Alfonso, A; Camilloni, G
PloS one
8
e83114
2013
Show Abstract
Sirtuins, class III histone deacetylases, are proteins homologous to the yeast protein Sir2p. Mammalian Sirt1 has been shown to be involved in energy metabolism, brain functions, inflammation and aging through its deacetylase activity, acting on both histone and non-histone substrates. In order to verify whether Sirt1 can replace Sir2p in the yeast cells, we expressed the full-length human Sirt1 protein in S.cerevisiae sir2Δ mutant strain. The structure of chromatin is basically maintained from yeast to human. Thus, yeast chromatin is a favourable environment to evaluate, inhibit or activate an ectopic histone deacetylase activity in an in vivo substrate. Mutant sir2Δ shows a series of different phenotypes, all dependent on the deacetylase activity of Sir2p. We analyzed the three silent loci where normally Sir2p acts: ribosomal DNA, telomeres and the mating type loci. Moreover, we verified extrachromosomal ribosomal DNA circles production and histone hyperacetylation levels, typical marks of sir2Δ strains. By strong SIRT1 overexpression in sir2Δ cells, we found that specific molecular phenotypes of the mutant revert almost to a wild-type condition. In particular, transcriptional silencing at rDNA was restored, extrachromosomal rDNA circles formation was repressed and histone acetylation at H3K9 and H4K16 decreased. The complementation at the other studied loci: HM loci, telomere and sub-telomere does not occur. Overall, our observations indicate that: i) SIRT1 gene is able to complement different molecular phenotypes of the sir2Δ mutant at rDNA ii) the in vivo screening of Sirt1 activity is possible in yeast. | 24349441
 |
Plant 45S rDNA clusters are fragile sites and their instability is associated with epigenetic alterations.
Huang, M; Li, H; Zhang, L; Gao, F; Wang, P; Hu, Y; Yan, S; Zhao, L; Zhang, Q; Tan, J; Liu, X; He, S; Li, L
PloS one
7
e35139
2012
Show Abstract
Our previous study demonstrated that 45S ribosomal DNA (45S rDNA) clusters were chromosome fragile sites expressed spontaneously in Lolium. In this study, fragile phenotypes of 45S rDNA were observed under aphidicolin (APH) incubation in several plant species. Further actinomycin D (ActD) treatment showed that transcriptional stress might interfere with chromatin packaging, resulting in 45S rDNA fragile expression. These data identified 45S rDNA sites as replication-dependent as well as transcription-dependent fragile sites in plants. In the presence of ActD, a dramatic switch to an open chromatin conformation and accumulated incomplete 5' end of the external transcribed spacer (5'ETS) transcripts were observed, accompanied by decreased DNA methylation, decreased levels of histone H3, and increased histone acetylation and levels of H3K4me2, suggesting that these epigenetic alterations are associated with failure of 45S rDNA condensation. Furthermore, the finding that γ-H2AX was accumulated at 45S rDNA sites following ActD treatment suggested that the DNA damage signaling pathway was associated with the appearance of 45S rDNA fragile phenotypes. Our data provide a link between 45S rDNA transcription and chromatin-packaging defects and open the door for further identifying the molecular mechanism involved. | 22509394
 |