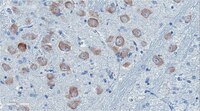
Immunolocalization of an amino-terminal fragment of apolipoprotein E in the Pick's disease brain.
Rohn, TT; Day, RJ; Catlin, LW; Brown, RJ; Rajic, AJ; Poon, WW
PloS one
8
e80180
2013
Show Abstract
Although the risk factor for apolipoprotein E (apoE) polymorphism in Alzheimer's disease (AD) has been well described, the role that apoE plays in other neurodegenerative diseases, including Pick's disease, is not well established. To examine a possible role of apoE in Pick's disease, an immunohistochemical analysis was performed utilizing a novel site-directed antibody that is specific for an amino-terminal fragment of apoE. Application of this antibody, termed the amino-terminal apoE cleavage fragment (nApoECF) antibody, consistently labeled Pick bodies within area CA1 of the hippocampus in 4 of the 5 cases examined. Co-localization of the nApoECF antibody with PHF-1, a general marker for Pick bodies, as well as with an antibody to caspase-cleaved tau (TauC3) was evident within the hippocampus. While staining of the nApoECF antibody was robust in area CA1, little co-localization with PHF-1 in Pick bodies within the dentate gyrus was observed. A quantitative analysis indicated that approximately 86% of the Pick bodies identified in area CA1 labeled with the nApoECF antibody. The presence of truncated apoE within Pick bodies suggests a broader role of apoE beyond AD and raises the question as to whether this protein contributes to pathogenesis associated with Pick's disease. | 24312462
 |
Identification of an amino-terminal fragment of apolipoprotein E4 that localizes to neurofibrillary tangles of the Alzheimer's disease brain.
Rohn, TT; Catlin, LW; Coonse, KG; Habig, JW
Brain research
1475
106-15
2012
Show Abstract
Although the risk factor for harboring the apolipoprotein E4 (apoE4) allele in late-onset Alzheimer's disease (AD) is well known, the mechanism by which apoE4 contributes to AD pathogenesis has yet to be clarified. Preferential cleavage of the ApoE4 isoform relative to other polymorphic forms appears to be significant, as the resulting fragments are associated with hallmarks of AD. To examine the possible role of apoE4 proteolysis in AD, we designed a site-directed antibody directed at position D172, which would yield a predicted amino-terminal fragment previously identified in AD brain extracts. Western blot analysis utilizing this novel antibody, termed the amino-terminal apoE4 cleavage fragment (nApoE4CF) Ab, consistently identified the predicted amino-terminal fragment (∼18kDa) in several commercially available forms of human recombinant apoE4 purified from E. coli. Mass spectrometry confirmed the identity of this 18kDa fragment as being an amino-terminal fragment of apoE4. Immunohistochemical experiments indicated the nApoE4CF Ab specifically labeled neurofibrillary tangles (NFTs) in AD frontal cortex sections that colocalized with the mature tangle marker PHF-1. Taken together, these results suggest a novel cleavage event of apoE4, generating an amino-terminal fragment that localizes within NFTs of the AD brain. | 22902767
 |