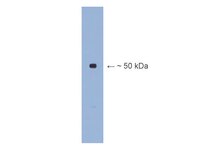
GluA2 mRNA distribution and regulation by miR-124 in hippocampal neurons.
Ho, VM; Dallalzadeh, LO; Karathanasis, N; Keles, MF; Vangala, S; Grogan, T; Poirazi, P; Martin, KC
Molecular and cellular neurosciences
61
1-12
2014
Show Abstract
AMPA-type glutamate receptors mediate fast, excitatory neurotransmission in the brain, and their concentrations at synapses are important determinants of synaptic strength. We investigated the post-transcriptional regulation of GluA2, the calcium-impermeable AMPA receptor subunit, by examining the subcellular distribution of its mRNA and evaluating its translational regulation by microRNA in cultured mouse hippocampal neurons. Using computational approaches, we identified a conserved microRNA-124 (miR-124) binding site in the 3'UTR of GluA2 and demonstrated that miR-124 regulated the translation of GluA2 mRNA reporters in a sequence-specific manner in luciferase assays. While we hypothesized that this regulation might occur in dendrites, our biochemical and fluorescent in situ hybridization (FISH) data indicate that GluA2 mRNA does not localize to dendrites or synapses of mouse hippocampal neurons. In contrast, we detected significant concentrations of miR-124 in dendrites. Overexpression of miR-124 in dissociated neurons results in a 30% knockdown of GluA2 protein, as measured by immunoblot and quantitative immunocytochemistry, without producing any changes in GluA2 mRNA concentrations. While total GluA2 concentrations are reduced, we did not detect any changes in the concentration of synaptic GluA2. We conclude from these results that miR-124 interacts with GluA2 mRNA in the cell body to downregulate translation. Our data support a model in which GluA2 is translated in the cell body and subsequently transported to neuronal dendrites and synapses, and suggest that synaptic GluA2 concentrations are modified primarily by regulated protein trafficking rather than by regulated local translation. | 24784359
 |
Generation of the SCN1A epilepsy mutation in hiPS cells using the TALEN technique.
Chen, W; Liu, J; Zhang, L; Xu, H; Guo, X; Deng, S; Liu, L; Yu, D; Chen, Y; Li, Z
Scientific reports
4
5404
2014
Show Abstract
Human induced pluripotent stem cells (iPSC) can be used to understand the pathological mechanisms of human disease. These cells are a promising source for cell-replacement therapy. However, such studies require genetically defined conditions. Such genetic manipulations can be performed using the novel Transcription Activator-Like Effector Nucleases (TALENs), which generate site-specific double-strand DNA breaks (DSBs) with high efficiency and precision. Combining the TALEN and iPSC methods, we developed two iPS cell lines by generating the point mutation A5768G in the SCN1A gene, which encodes the voltage-gated sodium channel Nav1.1 α subunit. The engineered iPSC maintained pluripotency and successfully differentiated into neurons with normal functional characteristics. The two cell lines differ exclusively at the epilepsy-susceptibility variant. The ability to robustly introduce disease-causing point mutations in normal hiPS cell lines can be used to generate a human cell model for studying epileptic mechanisms and for drug screening. | 24953032
 |
Protease-activated receptor-1 modulates hippocampal memory formation and synaptic plasticity.
Almonte, AG; Qadri, LH; Sultan, FA; Watson, JA; Mount, DJ; Rumbaugh, G; Sweatt, JD
Journal of neurochemistry
124
109-22
2013
Show Abstract
Protease-activated receptor-1 (PAR1) is an unusual G-protein coupled receptor (GPCR) that is activated through proteolytic cleavage by extracellular serine proteases. Although previous work has shown that inhibiting PAR1 activation is neuroprotective in models of ischemia, traumatic injury, and neurotoxicity, surprisingly little is known about PAR1's contribution to normal brain function. Here, we used PAR1-/- mice to investigate the contribution of PAR1 function to memory formation and synaptic function. We demonstrate that PAR1-/- mice have deficits in hippocampus-dependent memory. We also show that while PAR1-/- mice have normal baseline synaptic transmission at Schaffer collateral-CA1 synapses, they exhibit severe deficits in N-methyl-d-aspartate receptor (NMDAR)-dependent long-term potentiation (LTP). Mounting evidence indicates that activation of PAR1 leads to potentiation of NMDAR-mediated responses in CA1 pyramidal cells. Taken together, this evidence and our data suggest an important role for PAR1 function in NMDAR-dependent processes subserving memory formation and synaptic plasticity. | 23113835
 |