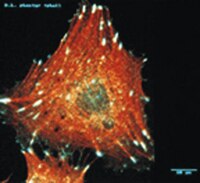
Adhesion, vitality and osteogenic differentiation capacity of adipose derived stem cells seeded on nitinol nanoparticle coatings.
Strauss, S; Neumeister, A; Barcikowski, S; Kracht, D; Kuhbier, JW; Radtke, C; Reimers, K; Vogt, PM
PloS one
8
e53309
2013
Show Abstract
Autologous cells can be used for a bioactivation of osteoimplants to enhance osseointegration. In this regard, adipose derived stem cells (ASCs) offer interesting perspectives in implantology because they are fast and easy to isolate. However, not all materials licensed for bone implants are equally suited for cell adhesion. Surface modifications are under investigation to promote cytocompatibility and cell growth. The presented study focused on influences of a Nitinol-nanoparticle coating on ASCs. Possible toxic effects as well as influences on the osteogenic differentiation potential of ASCs were evaluated by viability assays, scanning electron microscopy, immunofluorescence and alizarin red staining. It was previously shown that Nitinol-nanoparticles exert no cell toxic effects to ASCs either in soluble form or as surface coating. Here we could demonstrate that a Nitinol-nanoparticle surface coating enhances cell adherence and growth on Nitinol-surfaces. No negative influence on the osteogenic differentiation was observed. Nitinol-nanoparticle coatings offer new possibilities in implantology research regarding bioactivation by autologous ASCs, respectively enhancement of surface attraction to cells. | Immunofluorescence | 23308190
 |
Association between alphavbeta6 integrin expression, elevated p42/44 kDa MAPK, and plasminogen-dependent matrix degradation in ovarian cancer.
Ahmed, Nuzhat, et al.
J. Cell. Biochem., 84: 675-86 (2002)
2002
Show Abstract
Altered expression of alphav integrins plays a critical role in tumor growth, invasion, and metastasis. In this study, we show that normal human epithelial ovarian cell line, HOSE, and ovarian cancer cell lines, OVCA 429, OVCA 433, and OVHS-1, expressed alphav integrin and associated beta1, beta3, and beta5 subunits, but only ovarian cancer cell lines OVCA 429 and OVCA 433 expressed alphavbeta6 integrin. The expression of alphavbeta6 in OVCA 429 and OVCA 433 was far higher than alphavbeta3 and alphavbeta5 integrin and correlated with high p42/p44 mitogen activated protein kinase (MAPK) activity and high secretion of high molecular weight urokinase plasminogen activator (HMW-uPA), pro-metalloproteinase 2 and 9 (pro-MMP-9 and pro-MMP-2). In contrast to HOSE and OVHS 1, OVCA 433 and OVCA 429 exhibited approximately 2-fold more plasminogen-dependent [3H]-collagen type IV degradation. Plasminogen-dependent [3H]-collagen IV degradation was inhibited by inhibitor of uPA (amiloride) and MMP (phenanthroline) and by antibodies against uPA or MMP-9 or alphavbeta6 integrin, indicating the involvement of alphavbeta6 integrin, uPA and MMP-9 in the process. The alphavbeta6 correlated increase in HMW-uPA and pro-MMP secretion could be inhibited by tyrosine kinase inhibitor genistein or the MEK 1 inhibitor U0126, consistent with a role of active p42/44 MAPK in the elevation of uPA, MMP-9, and MMP-2 secretion. Under similar conditions, genistein and U0126 inhibited plasminogen-dependent [3H]-collagen type IV degradation. These data suggest that sustained elevation of p42/44 MAPK activity may be required for the co-expression of alphavbeta6 integrin, which in turn may regulate the malignant potential of ovarian cancer cells via proteolytic mechanisms. | | 11835393
 |
Overexpression of alpha(v)beta6 integrin in serous epithelial ovarian cancer regulates extracellular matrix degradation via the plasminogen activation cascade.
Ahmed, N, et al.
Carcinogenesis, 23: 237-44 (2002)
2002
Show Abstract
Recent evidence suggests that integrins are involved in the multi-step process of tumour metastasis. The biological relevance of alpha(v) integrins and associated beta-subunits in ovarian cancer metastasis was examined by analysing the expression of these cell surface receptors in nine ovarian cancer cell lines and also in the primary human ovarian surface epithelial cell line (HOSE). beta1, beta3 and beta5 subunits were present in all ten ovarian cell lines. beta6 subunit was present at varying levels in eight out of nine cancer cell lines but was absent in the HOSE cell line. Immunohistochemical staining showed that beta6 was present in both non-invasive (borderline) and high-grade ovarian cancer tissues but was absent in benign and normal ovarian tissue. High alpha(v)beta6 integrin expressing ovarian cancer cell lines had high cell surface expression of uPA and uPAR. Ovarian cancer cell lines expressing high to moderate level of alpha(v)beta6 integrin demonstrated ligand-independent enhanced levels of high molecular weight (HMW)-uPA and pro-matrix metalloproteinase 2 and 9 (pro-MMP-2 and pro-MMP-9) expression in the tumour-conditioned medium. High and moderate expression of alpha(v)beta6 integrin correlated with increased plasminogen-dependent degradation of extracellular matrix which could be inhibited by inhibitors of plasmin, uPA and MMPs or by monoclonal antibody against uPA, MMP-9 or alpha(v)beta6 integrin. These results suggest that endogenous de novo expression of alpha(v)beta6 integrin in ovarian cancer cells may contribute to their invasive potential, and that alpha(v)beta6 expression may play a role in ovarian cancer progression and metastasis. | | 11872628
 |
Different integrins mediate cell spreading, haptotaxis and lateral migration of HaCaT keratinocytes on fibronectin.
L Koivisto, K Larjava, L Häkkinen, V J Uitto, J Heino, H Larjava
Cell adhesion and communication
7
245-57
1999
Show Abstract
Collaborative role of various fibronectin-binding integrins (alpha5beta1, alphavbeta1 and alphavbeta6) as mediators of cell adhesion and migration on fibronectin was studied using cultured HaCaT keratinocytes. This cell line spontaneously expressed all three fibronectin-binding integrins. In addition, the expression of alphavbeta6 integrin was strongly and specifically upregulated by transforming growth factor-beta1 (TGFbeta1) whereas the amount of other integrins remained practically unchanged on the cell surface. Adhesion, spreading and motility of HaCaT keratinocytes on fibronectin were promoted by TGFbeta1. Based on antibody blocking experiments, both untreated and TGFbeta1-treated HaCaT cells used alphavbeta6 integrin as their main fibronectin receptor for cell spreading. In contrast to TGFbeta1-treated cells, the untreated cells also needed alpha5beta1 integrin for maximal cell spreading on fibronectin. Combinations of antibodies blocking both of these receptors totally prevented spreading of both untreated and TGFbeta1-treated cells. Haptotactic motility of individual HaCaT cells through fibronectin-coated membranes was again mainly dependent on alphavbeta6 integrin, while alphavbeta1 and alpha5beta1 integrins played a lesser role both in untreated and TGFbeta1-treated HaCaT cells. However, unlike haptotaxis, lateral migration of HaCaT cell sheet was mainly mediated by beta1 integrins, and alphavbeta6 integrin showed a minor role. The migration process appeared to involve a number of beta1 integrins that could adaptively replace each other when blocking antibodies were present. Thus, keratinocytes appear to use different fibronectin receptors for different functions, such as cell spreading, haptotaxis and lateral migration. The cells can also adapt to a situation where one receptor is unfunctional by switching to another receptor of the same ligand. | | 10626908
 |
Integrins beta 5, beta 3 and alpha v are apically distributed in endometrial epithelium.
Aplin, J D, et al.
Mol. Hum. Reprod., 2: 527-34 (1996)
1996
Show Abstract
Several adhesion molecules have been shown to occur at the surface of endometrial cells. One of these is the integrin alpha v subunit which associates with various beta chains including beta 5. We demonstrate the presence of integrin beta 5 polypeptide in human endometrial epithelial cells throughout the menstrual cycle using immunocytochemistry with monospecific antibodies, and at the mRNA level by thermal amplification from endometrial cDNA. Integrin beta 5 is also found in a population of bone marrow-derived cells. A notable feature of the distribution of the beta 5 subunit in the glandular and luminal epithelium is its apical localization, which may suggest an involvement in implantation. However, no evidence was found for regulated expression of epithelial beta 5. In mouse, the beta 5 subunit is found at both the apical and basal surface of epithelial cells and expression is essentially oestrous cycle-independent. Comparisons are made in both species with the distribution of the alpha v and beta 3 subunits which also localize to the apical epithelium. | | 9239663
 |
Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor.
Cheresh, D A
Proc. Natl. Acad. Sci. U.S.A., 84: 6471-5 (1987)
1987
Show Abstract
Human umbilical vein endothelial cells express a heterodimeric adhesion receptor complex consisting of noncovalently associated alpha and beta subunits that under reducing conditions have molecular masses of 135 kDa and 115 kDa, respectively. This complex can be isolated in pure form from an affinity matrix consisting of an Arg-Gly-Asp-containing heptapeptide and is specifically immunoprecipitated with monoclonal antibodies (mAbs) directed against the vitronectin receptor of human melanoma cells. These data suggest that this complex is one member of a large family of cell adhesion receptors. One of the mAbs, LM609, inhibits the attachment of human endothelial cells to fibrinogen, von Willebrand factor, and vitronectin yet has no effect on the attachment of these cells to fibronectin, collagen, or laminin. In addition, mAb LM609 inhibits attachment of endothelial cells to an immobilized synthetic peptide containing the Arg-Gly-Asp sequence. This adhesion receptor appears structurally similar to the IIb/IIIa glycoprotein complex expressed on platelets yet is antigenically distinct, since mAb LM609 fails to recognize IIb/IIIa glycoproteins. This receptor organizes in clusters on endothelial cells during their attachment to von Willebrand factor, vitronectin, or the Arg-Gly-Asp-containing heptapeptide. The data presented in this report suggest that Arg-Gly-Asp recognition may play a significant role in biological events associated with vascular proliferation. | | 2442758
 |