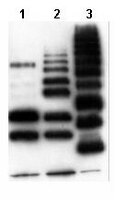
Poly-ADP ribosylation of PTEN by tankyrases promotes PTEN degradation and tumor growth.
Li, N; Zhang, Y; Han, X; Liang, K; Wang, J; Feng, L; Wang, W; Songyang, Z; Lin, C; Yang, L; Yu, Y; Chen, J
Genes & development
29
157-70
2015
Show Abstract
PTEN [phosphatidylinositol (3,4,5)-trisphosphate phosphatase and tensin homolog deleted from chromosome 10], a phosphatase and critical tumor suppressor, is regulated by numerous post-translational modifications, including phosphorylation, ubiquitination, acetylation, and SUMOylation, which affect PTEN localization and protein stability. Here we report ADP-ribosylation as a new post-translational modification of PTEN. We identified PTEN as a novel substrate of tankyrases, which are members of the poly(ADP-ribose) polymerases (PARPs). We showed that tankyrases interact with and ribosylate PTEN, which promotes the recognition of PTEN by a PAR-binding E3 ubiquitin ligase, RNF146, leading to PTEN ubiquitination and degradation. Double knockdown of tankyrase1/2 stabilized PTEN, resulting in the subsequent down-regulation of AKT phosphorylation and thus suppressed cell proliferation and glycolysis in vitro and tumor growth in vivo. Furthermore, tankyrases were up-regulated and negatively correlated with PTEN expression in human colon carcinomas. Together, our study revealed a new regulation of PTEN and highlighted a role for tankyrases in the PTEN-AKT pathway that can be explored further for cancer treatment. | | 25547115
 |
Characterization of aggregate/aggresome structures formed by polyhedrin of Bombyx mori nucleopolyhedrovirus.
Guo, ZJ; Tao, LX; Dong, XY; Yu, MH; Tian, T; Tang, XD
Scientific reports
5
14601
2015
Show Abstract
Virus infections often lead to formation of aggregates and aggresomes in host cells. In this study, production of aggregates and aggresomes by the highly expressed protein polyhedrin of Bombyx mori nucleopolyhedrovirus (BmNPV) at 24 h postinfection (p.i.) was detected with a fluorescent molecular dye, and verified by colocalization of polyhedrin with aggresomal markers, GFP-250 and γ-tubulin. Polyhedrin aggregates showed hallmark characteristics of aggresomes: formation was microtubule-dependent; they colocalized with heat shock cognates/proteins of the 70-kDa family (HSC/HSP70s), ubiquitinated proteins and recruited the mitochondria. Aggregated polyhedrin protein gradually gained its active conformation accompanying progress of BmNPV infection. At 48 h p.i. recovered polyhedrin bound directly to Bombyx mori microtubule-associated protein 1-light chain 3 (BmLC3), an autophagosome marker, and was colocalized with BmLC3 to the isolation membrane of autophagosome, implying the involvement of polyhedrin in cellular autophagy. Inhibition of autophagy by 3-methyladenine (3-MA) dramatically resulted in decrease of polyhedrin expression and polyhedra particle production. These observations suggested that highly expressed polyhedrin forms aggregate to get involved in cellular autophagy then play an important role in polyhedra production. | | 26440217
 |
Tax posttranslational modifications and interaction with calreticulin in MT-2 cells and human peripheral blood mononuclear cells of human T cell lymphotropic virus type-I-associated myelopathy/tropical spastic paraparesis patients.
Medina, F; Quintremil, S; Alberti, C; Barriga, A; Cartier, L; Puente, J; Ramírez, E; Ferreira, A; Tanaka, Y; Valenzuela, MA
AIDS research and human retroviruses
30
370-9
2014
Show Abstract
The human retrovirus human T cell lymphotropic virus type-I (HTLV-1) is the etiologic agent of HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). Axonal degeneration in HAM/TSP patients occurs without neuron infection, with the secreted viral Tax protein proposed to be involved. We previously found that Tax secreted into the culture medium of MT-2 cells (HTLV-1-infected cell line) produced neurite retraction in neuroblastoma cells differentiated to neuronal type. To assess the relevance of Tax posttranslational modifications on this effect, we addressed the question of whether Tax secreted by MT-2 cells and peripheral blood mononuclear cells (PBMCs) of HTLV-1-infected subjects is modified. The interaction of Tax with calreticulin (CRT) that modulates intracellular Tax localization and secretion has been described. We studied Tax localization and modifications in MT-2 cells and its interaction with CRT. Intracellular Tax in MT-2 cells was assessed by flow cytometry, corresponding mainly to a 71-kDa protein followed by western blot. This protein reported as a chimera with gp21 viral protein-confirmed by mass spectrometry-showed no ubiquitination or SUMOylation. The Tax-CRT interaction was determined by confocal microscopy and coimmunoprecipitation. Extracellular Tax from HAM/TSP PBMCs is ubiquitinated according to western blot, and its interaction with CRT was shown by coimmunoprecipitation. A positive correlation between Tax and CRT secretion was observed in HAM/TSP PBMCs and asymptomatic carriers. For both proteins inhibitors and activators of secretion showed secretion through the endoplasmic reticulum-Golgi complex. Tax, present in PBMC culture medium, produced neurite retraction in differentiated neuroblastoma cells. These results suggest that Tax, whether ubiquitinated or not, is active for neurite retraction. | Western Blotting | 24321043
 |
Nuclear ubiquitination by FBXL5 modulates Snail1 DNA binding and stability.
Viñas-Castells, R; Frías, Á; Robles-Lanuza, E; Zhang, K; Longmore, GD; García de Herreros, A; Díaz, VM
Nucleic acids research
42
1079-94
2014
Show Abstract
The zinc finger transcription factor Snail1 regulates epithelial to mesenchymal transition, repressing epithelial markers and activating mesenchymal genes. Snail1 is an extremely labile protein degraded by the cytoplasmic ubiquitin-ligases β-TrCP1/FBXW1 and Ppa/FBXL14. Using a short hairpin RNA screening, we have identified FBXL5 as a novel Snail1 ubiquitin ligase. FBXL5 is located in the nucleus where it interacts with Snail1 promoting its polyubiquitination and affecting Snail1 protein stability and function by impairing DNA binding. Snail1 downregulation by FBXL5 is prevented by Lats2, a protein kinase that phosphorylates Snail1 precluding its nuclear export but not its polyubiquitination. Actually, although polyubiquitination by FBXL5 takes place in the nucleus, Snail1 is degraded in the cytosol. Finally, FBXL5 is highly sensitive to stress conditions and is downregulated by iron depletion and γ-irradiation, explaining Snail1 stabilization in these conditions. These results characterize a novel nuclear ubiquitin ligase controlling Snail1 protein stability and provide the molecular basis for understanding how radiotherapy upregulates the epithelial to mesenchymal transition-inducer Snail1. | | 24157836
 |
Normal autophagic activity in macrophages from mice lacking Gαi3, AGS3, or RGS19.
Vural, A; McQuiston, TJ; Blumer, JB; Park, C; Hwang, IY; Williams-Bey, Y; Shi, CS; Ma, DZ; Kehrl, JH
PloS one
8
e81886
2013
Show Abstract
In macrophages autophagy assists antigen presentation, affects cytokine release, and promotes intracellular pathogen elimination. In some cells autophagy is modulated by a signaling pathway that employs Gαi3, Activator of G-protein Signaling-3 (AGS3/GPSM1), and Regulator of G-protein Signaling 19 (RGS19). As macrophages express each of these proteins, we tested their importance in regulating macrophage autophagy. We assessed LC3 processing and the formation of LC3 puncta in bone marrow derived macrophages prepared from wild type, Gnai3(-/-), Gpsm1(-/-), or Rgs19(-/-) mice following amino acid starvation or Nigericin treatment. In addition, we evaluated rapamycin-induced autophagic proteolysis rates by long-lived protein degradation assays and anti-autophagic action after rapamycin induction in wild type, Gnai3(-/-), and Gpsm1(-/-) macrophages. In similar assays we compared macrophages treated or not with pertussis toxin, an inhibitor of GPCR (G-protein couple receptor) triggered Gαi nucleotide exchange. Despite previous findings, the level of basal autophagy, autophagic induction, autophagic flux, autophagic degradation and the anti-autophagic action in macrophages that lacked Gαi3, AGS3, or RGS19; or had been treated with pertussis toxin, were similar to controls. These results indicate that while Gαi signaling may impact autophagy in some cell types it does not in macrophages. | | 24312373
 |
Sequestration of CDH1 by MAD2L2 prevents premature APC/C activation prior to anaphase onset.
Listovsky, T; Sale, JE
The Journal of cell biology
203
87-100
2013
Show Abstract
The switch from activation of the anaphase-promoting complex/cyclosome (APC/C) by CDC20 to CDH1 during anaphase is crucial for accurate mitosis. APC/C(CDC20) ubiquitinates a limited set of substrates for subsequent degradation, including Cyclin B1 and Securin, whereas APC/C(CDH1) has a broader specificity. This switch depends on dephosphorylation of CDH1 and the APC/C, and on the degradation of CDC20. Here we show, in human cells, that the APC/C inhibitor MAD2L2 also contributes to ensuring the sequential activation of the APC/C by CDC20 and CDH1. In prometaphase, MAD2L2 sequestered free CDH1 away from the APC/C. At the onset of anaphase, MAD2L2 was rapidly degraded by APC/C(CDC20), releasing CDH1 to activate the dephosphorylated APC/C. Loss of MAD2L2 led to premature association of CDH1 with the APC/C, early destruction of APC/C(CDH1) substrates, and accelerated mitosis with frequent mitotic aberrations. Thus, MAD2L2 helps to ensure a robustly bistable switch between APC/C(CDC20) and APC/C(CDH1) during the metaphase-to-anaphase transition, thereby contributing to mitotic fidelity. | | 24100295
 |
Ubiquitination of the N-terminal region of caveolin-1 regulates endosomal sorting by the VCP/p97 AAA-ATPase.
Kirchner, P; Bug, M; Meyer, H
The Journal of biological chemistry
288
7363-72
2013
Show Abstract
Caveolin-1 (CAV1) is the defining constituent of caveolae at the plasma membrane of many mammalian cells. For turnover, CAV1 is ubiquitinated and sorted to late endosomes and lysosomes. Sorting of CAV1 requires the AAA+-type ATPase VCP and its cofactor UBXD1. However, it is unclear in which region CAV1 is ubiquitinated and how ubiquitination is linked to sorting of CAV1 by VCP-UBXD1. Here, we show through site-directed mutagenesis that ubiquitination of CAV1 occurs at any of the six lysine residues, 5, 26, 30, 39, 47, and 57, that are clustered in the N-terminal region but not at lysines in the oligomerization, intramembrane, or C-terminal domains. Mutation of Lys-5-57 to arginines prevented binding of the VCP-UBXD1 complex and, importantly, strongly reduced recruitment of VCP-UBXD1 to endocytic compartments. Moreover, the Lys-5-57Arg mutation specifically interfered with trafficking of CAV1 from early to late endosomes. Conversely and consistently, depletion of VCP or UBXD1 led to accumulation of ubiquitinated CAV1, suggesting that VCP acts downstream of ubiquitination and is required for transport of the ubiquitinated form of CAV1 to late endosomes. These results define the N-terminal region of CAV1 as the critical ubiquitin conjugation site and, together with previous data, demonstrate the significance of this ubiquitination for binding to the VCP-UBXD1 complex and for sorting into lysosomes. | | 23335559
 |
Regulation of the DNA damage response on male meiotic sex chromosomes.
Lu, LY; Xiong, Y; Kuang, H; Korakavi, G; Yu, X
Nature communications
4
2105
2013
Show Abstract
During meiotic prophase in males, the sex chromosomes partially synapse to form the XY body, a unique structure that recruits proteins involved in the DNA damage response, which is believed to be important for silencing of the sex chromosomes. It remains elusive how the DNA damage response in the XY body is regulated. Here we show that H2AX-MDC1-RNF8 signaling, which is well characterized in somatic cells, is dispensable for the recruitment of proteins to the unsynapsed axes in the XY body. On the other hand, the DNA damage response that spreads over the sex chromosomes is largely similar to that in somatic cells. This analysis shows that there are important differences between the regulation of the DNA damage response at the XY body and at DNA damage sites in somatic cells. | | 23812044
 |
Lysosome-mediated processing of chromatin in senescence.
Ivanov, A; Pawlikowski, J; Manoharan, I; van Tuyn, J; Nelson, DM; Rai, TS; Shah, PP; Hewitt, G; Korolchuk, VI; Passos, JF; Wu, H; Berger, SL; Adams, PD
The Journal of cell biology
202
129-43
2013
Show Abstract
Cellular senescence is a stable proliferation arrest, a potent tumor suppressor mechanism, and a likely contributor to tissue aging. Cellular senescence involves extensive cellular remodeling, including of chromatin structure. Autophagy and lysosomes are important for recycling of cellular constituents and cell remodeling. Here we show that an autophagy/lysosomal pathway processes chromatin in senescent cells. In senescent cells, lamin A/C-negative, but strongly γ-H2AX-positive and H3K27me3-positive, cytoplasmic chromatin fragments (CCFs) budded off nuclei, and this was associated with lamin B1 down-regulation and the loss of nuclear envelope integrity. In the cytoplasm, CCFs were targeted by the autophagy machinery. Senescent cells exhibited markers of lysosomal-mediated proteolytic processing of histones and were progressively depleted of total histone content in a lysosome-dependent manner. In vivo, depletion of histones correlated with nevus maturation, an established histopathologic parameter associated with proliferation arrest and clinical benignancy. We conclude that senescent cells process their chromatin via an autophagy/lysosomal pathway and that this might contribute to stability of senescence and tumor suppression. | Immunofluorescence | 23816621
 |
A two-step mechanism for TRF2-mediated chromosome-end protection.
Okamoto, K; Bartocci, C; Ouzounov, I; Diedrich, JK; Yates, JR; Denchi, EL
Nature
494
502-5
2013
Show Abstract
Mammalian telomeres repress DNA-damage activation at natural chromosome ends by recruiting specific inhibitors of the DNA-damage machinery that form a protective complex termed shelterin. Within this complex, TRF2 (also known as TERF2) has a crucial role in end protection through the suppression of ATM activation and the formation of end-to-end chromosome fusions. Here we address the molecular properties of TRF2 that are both necessary and sufficient to protect chromosome ends in mouse embryonic fibroblasts. Our data support a two-step mechanism for TRF2-mediated end protection. First, the dimerization domain of TRF2 is required to inhibit ATM activation, the key initial step involved in the activation of a DNA-damage response (DDR). Next, TRF2 independently suppresses the propagation of DNA-damage signalling downstream of ATM activation. This novel modulation of the DDR at telomeres occurs at the level of the E3 ubiquitin ligase RNF168 (ref. 3). Inhibition of RNF168 at telomeres involves the deubiquitinating enzyme BRCC3 and the ubiquitin ligase UBR5, and is sufficient to suppress chromosome end-to-end fusions. This two-step mechanism for TRF2-mediated end protection helps to explain the apparent paradox of frequent localization of DDR proteins at functional telomeres without concurrent induction of detrimental DNA-repair activities. | | 23389450
 |