SCM121 Sigma-AldrichOsteoMAX-XF™ Differentiation Medium
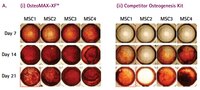
Defined Xeno-Free osteogenesis differentiation medium that is specially formulated to readily differentiate human mesenchymal stem cells (MSCs) into mature osteocytes as assessed by alizarin red & alkaline phosphatase staining.
More>> Defined Xeno-Free osteogenesis differentiation medium that is specially formulated to readily differentiate human mesenchymal stem cells (MSCs) into mature osteocytes as assessed by alizarin red & alkaline phosphatase staining. Less<<Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| Key Applications |
|---|
| CULT, Cult |
| References |
|---|
| Product Information | |
|---|---|
| Components |
|
| HS Code | 3002 15 90 |
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Cross Reactivity | None |
| Media Form | Liquid |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Packaging Information | |
|---|---|
| Material Size | 100ml/kit |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| SCM121 | 04053252847899 |
Documentation
OsteoMAX-XF™ Differentiation Medium SDS
| Title |
|---|
Technical Info
| Title |
|---|
| A Novel Serum-free and Xeno-free Differentiation Medium for Accelerated Osteogenic Differentiation of Human Mesenchymal Stem Cells |
User Guides
| Title |
|---|
| OsteoMAX-XFTM Differentiation Medium |