648469 Sigma-AldrichTroglitazone - CAS 97322-87-7 - Calbiochem
A α-tocopherol (vitamin E) moiety containing thiazolidinedione class of insulin-sensitizer that acts as an activator of peroxisome proliferator-activated receptors γ (PPARγ).
More>> A α-tocopherol (vitamin E) moiety containing thiazolidinedione class of insulin-sensitizer that acts as an activator of peroxisome proliferator-activated receptors γ (PPARγ). Less<<Recommended Products
Overview
| Replacement Information |
|---|
Key Specifications Table
| CAS # | Empirical Formula |
|---|---|
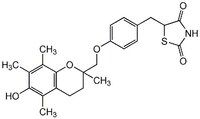
| 97322-87-7 | C₂₄H₂₇NO₅S |
Pricing & Availability
| Catalog Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 648469-5MG |
|
Plastic ampoule | 5 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 97322-87-7 |
| ATP Competitive | N |
| Form | White to yellow solid |
| Hill Formula | C₂₄H₂₇NO₅S |
| Chemical formula | C₂₄H₂₇NO₅S |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | Peroxisome proliferator-activated receptors γ (PPARγ) |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | N |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | XJ5813130 |
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalog Number | GTIN |
| 648469-5MG | 04055977262469 |
Documentation
Troglitazone - CAS 97322-87-7 - Calbiochem SDS
| Title |
|---|
Troglitazone - CAS 97322-87-7 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 648469 |
References
| Reference overview |
|---|
| Masamune, A., et al. 2002. J. Biol. Chem. 277, 141. Boyault, S., et al. 2001. FEBS Lett. 501, 24. de Dios, S.T., et al. 2001. J. Diabetes Complications 15, 120. Furuse, Y., et al. 2001. Br. J. Pharmacol. 133, 1307. Ghanim, H., et al. 2001. J. Clin. Endocrinol. Metab. 86, 1306. Goetze, S., et al. 2001. J. Cardiovasc. Pharmacol. 38, 909. Koga, H., et al. 2001. Hepatology 33, 1087. Rosen, E.D. and Spiegelman, B.M., 2001. J. Biol. Chem. 276, 37731. Subbaramaiah, K., et al. 2001. J. Biol. Chem. 276, 12440. Takashima, T., et al. 2001. Int. J. Oncol. 19, 465. Takeda, K., et al. 2001. J. Biol. Chem. 276, 48950. Tanaka, T., et al. 2001. Cancer Res. 61, 2424. Wakino, S., et al. 2001. J. Biol. Chem. 276, 47650. |