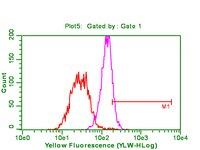
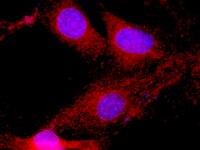
PDK1, one of the missing links in insulin signal transduction?
Cohen, P, et al.
FEBS Lett., 410: 3-10 (1997)
1997
Show Abstract
The initial steps in insulin signal transduction occur at the plasma membrane and lead to the activation of phosphatidylinositide (PtdIns) 3-kinase and the formation of PtdIns(3,4,5,)P3 in the inner leaflet of the plasma membrane which is then converted to PtdIns(3,4)P2 by a specific phosphatase. Inhibitors of PtdIns 3-kinase suppress nearly all the metabolic actions of insulin indicating that PtdIns(3,4,5)P3 and/or PtdIns(3,4)P2 are key 'second messengers' for this hormone. A major effect of insulin is its ability to stimulate the synthesis of glycogen in skeletal muscle. By 'working backwards' from glycogen synthesis, we have dissected an insulin-stimulated protein kinase cascade which is triggered by the activation of PtdIns 3-kinase. The first enzyme in this cascade is termed 3-phosphoinositide-dependent protein kinase (PDK1), because it is only active in the presence of PtdIns(3,4,5)P3 or PtdIns(3,4)P2. PDK1 then activates protein kinase B (PKB) which, in turn, inactivates glycogen synthase kinase-3 (GSK3), leading to the dephosphorylation and activation of glycogen synthase and hence to an acceleration of glycogen synthesis. We review the evidence which indicates that the phosphorylation of other proteins by PKB and GSK3 is likely to mediate many of the intracellular actions of insulin. | 9247112
 |